Effects of dietary forage-to-concentrate ratio on nutrient digestibility and enteric methane production in growing goats (Capra hircus hircus) and Sika deer (Cervus nippon hortulorum)
Article information
Abstract
Objective
Two experiments were conducted to determine the effects of forage-to-concentrate (F:C) ratio on the nutrient digestibility and enteric methane (CH4) emission in growing goats and Sika deer.
Methods
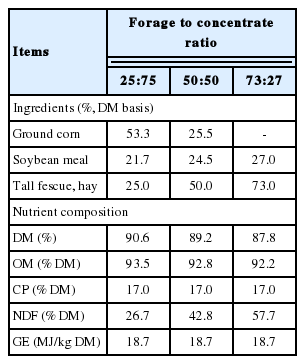
Three male growing goats (body weight [BW] = 19.0±0.7 kg) and three male growing deer (BW = 19.3±1.2 kg) were respectively allotted to a 3×3 Latin square design with an adaptation period of 7 d and a data collection period of 3 d. Respiration-metabolism chambers were used for measuring the enteric CH4 emission. Treatments of low (25:75), moderate (50:50), and high (73:27) F:C ratios were given to both goats and Sika deer.
Results
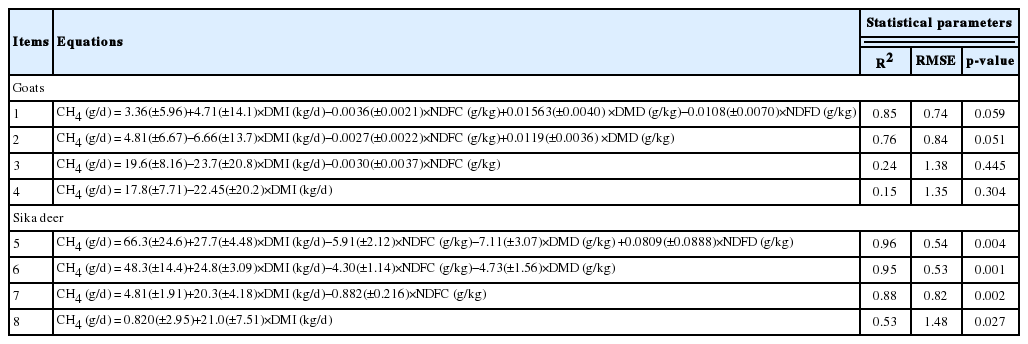
Dry matter (DM) and organic matter (OM) digestibility decreased linearly with increasing F:C ratio in both goats and Sika deer. In both goats and Sika deer, the CH4 emissions expressed as g/d, g/kg BW0.75, % of gross energy intake, g/kg DM intake (DMI), and g/kg OM intake (OMI) decreased linearly as the F:C ratio increased, however, the CH4 emissions expressed as g/kg digested DMI and OMI were not affected by the F:C ratio. Eight equations were derived for predicting the enteric CH4 emission from goats and Sika deer. For goat, equation 1 was found to be of the highest accuracy: CH4 (g/d) = 3.36+4.71×DMI (kg/d)−0.0036×neutral detergent fiber concentrate (NDFC, g/kg)+0.01563×dry matter digestibility (DMD, g/kg)−0.0108×neutral detergent fiber digestibility (NDFD, g/kg). For Sika deer, equation 5 was found to be of the highest accuracy: CH4 (g/d) = 66.3+27.7×DMI (kg/d)−5.91×NDFC (g/kg)−7.11× DMD (g/kg)+0.0809×NDFD (g/kg).
Conclusion
Digested nutrient intake could be considered when determining the CH4 generation factor in goats and Sika deer. Finally, the enteric CH4 prediction model for goats and Sika deer were estimated.
INTRODUCTION
Methane (CH4) production by enteric fermentation in ruminants is recognized as one of the major sources of greenhouse gas emissions worldwide [1]. Besides, the enteric CH4 represents an energy loss, ranging from 2% to 12% of the gross energy intake, for an animal [2]. Generally, the enteric CH4 production by ruminants is affected by various dietary factors such as the level of intake [3], carbohydrate type [4,5], forage processing [6], fat addition [7], and ionophore addition [8]. Moreover, the forage-to-concentrate (F:C) ratio in diets affects nutrient digestibility and enteric CH4 emission in many ruminants [9–11]. Although cattle and buffalo produce maximum greenhouse gases, 4.4% of the total greenhouse gas emissions from the livestock sector is contributed by the goats worldwide [12]. In addition, a large number of Sika deer inhabit East Asian areas or are domesticated in some of those areas [13]. Many studies have been conducted for measuring the emission of enteric CH4 from dairy cattle, beef cattle, or sheep; however, only a few studies have been conducted for goats and Sika deer.
Mathematical models have been developed for predicting the enteric CH4 production in ruminants [3,14,15]. Although the models based on databases taken from different studies for enteric CH4 emission from goats were already developed [16], to our knowledge, the model for enteric CH4 emission from deer did not exist. Therefore, the objective of the present study was to determine the effects of the F:C ratio on the nutrient digestibility and enteric CH4 emission from growing goats and Sika deer as well as to derive the equations for CH4 production.
MATERIALS AND METHODS
Two experiments were conducted to determine the nutrient digestibility and emission of enteric CH4 and CO2 in goats (Capra hircus hircus) and Sika deer (Cervus nippon hortulorum). We performed the experimental procedures in accordance with the Institutional Animal Care and Use Committee of Konkuk University.
Animals, diets, and experimental design
Three growing male goats with initial body weight (BW) of 19.0± 0.7 kg and three growing male deer with initial BW of 19.3±1.2 kg were used. Experiments were conducted in an environmentally controlled room (20°C±3°C). Each animal was housed individually in a respiration-metabolism chamber described by Li et al [17]. Experimental diets based on 2% of initial BW (dry matter [DM] basis) were fed daily at 1100 h. Water and mineral blocks were provided at all times. Orts were removed daily and weighed at 1000 h for DM intake calculation. Fecal samples were collected everyday by using the total collection method, dried immediately, and stored at −20°C for subsequent chemical analysis. Three experimental diets were prepared for both goat and deer experiments (Table 1). The dietary treatments included low (25:75), moderate (50:50), and high (73:27) F:C ratios. The experimental design consisted of a 3×3 Latin square design with a diet adaptation period of 7 d and a data collection period of 3 days. Adaption period was according to literature reference [18,19]. The animals were weighed at the beginning of each period.
Chemical analysis
All ingredients and fecal samples were analyzed in duplicate for DM, organic matter (OM), crude protein (CP), and ether extract (EE) as described by AOAC [20]. The contents of neutral detergent fiber (NDF) were analyzed using heat stable α-amylase (Sigma A3306; Sigma Chemical Co., St. Louis, MO, USA) according to the method described by Van Soest et al [21]. Gross energy (GE) was determined using a bomb calorimeter (C5000; IKA, Staufen, Germany).
Gas production measurement
The CH4 and carbon dioxide (CO2) production were measured using a respiration-metabolism chamber system [17]. A recovery test was performed before each period using standard CH4 gas (1.67%, v/v). Inlet and outlet gases were measured by a gas flow meter (GFM57, Aalborg Instruments & Controls Inc., Orangeburg, NY, USA); a sample pump (Columbus Instruments, Columbus, OH, USA) was used to collect gas samples. The gas samples were passed through a desiccant composed of calcium sulfate (CaSO4), before the samples flew into the gas analyzer. Non-dispersive infrared gas analyzer (VA-3000; Horiba Stec Co., Kyoto, Japan) was used to analyze the CH4 and CO2 concentrates.
Statistical analysis
The data were analyzed using SAS PROC MIXED (Version 9.2; SAS Institute Inc., Cary, NC, USA). The model considered the diet as the fixed effect and both animals and periods as the random effects. Orthogonal contrasts for linear and quadratic effects were performed with polynomials determined by SAS PROC IML (Version 9.2; SAS Institute Inc., USA). All data were presented as the least squares means. Treatment effects were considered significant at p<0.05, and trends were considered at 0.05≤p<0.10. The SAS PROC REG (Version 9.2; SAS Institute Inc., USA) was used for estimating the simple and multiple linear equations. Equations were evaluated on the basis of root mean square error (RMSE), adjusted-R2, and p-value.
RESULTS AND DISCUSSION
The DM and OM digestibility of goats decreased linearly (p<0.01) as the F:C ratio increased (Table 2). The DM, OM, and CP digestibility of Sika deer also decreased linearly (p<0.01) as the F:C ratio increased. An increase in the F:C ratio decreases the DM and OM digestibility for other ruminants, such as a cow [22] and sheep [23,24], because the forage has a generally higher NDF content than the concentrate. As structural carbohydrates (e.g. NDF) are usually less digestible than non-fiber carbohydrates, the total digestibility decreases with increasing proportions of forage in the diet [4]. In agreement with previously reported results in other studies on goats [25,26] or deer [27], in the present study, the DM and OM digestibility decreased (p<0.01) with increasing F:C ratios. As the NDF digestibility of goats and Sika deer were not significantly affected by the F:C ratios, their DM digestibility decreased with increasing F:C ratios.
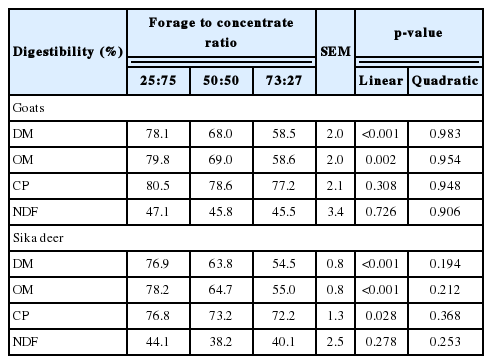
Effect of forage to concentrate ratio on nutrient digestibility in goats (Capra hircus hircus) and Sika deer (Cervus nippon hortulorum)
In goats and Sika deer, the enteric emission of CH4 expressed as g/d, g/kg BW0.75, % of gross energy intake (GEI), g/kg DMI, and g/kg OMI decreased linearly (p<0.05) with increasing F:C ratios (Table 3). However, no difference was observed in enteric CH4 production expressed as g/kg digested dry matter intake (DDMI) and g/kg digested organic matter intake (DOMI) in both goats and Sika deer. In goats, the CO2 production expressed as g/kg BW0.75 decreased linearly (p<0.05) with increasing F:C ratio, and there was a tendency (p = 0.078) for a decrease in the CO2 production expressed as g/d. The emission of enteric CO2 by Sika deer decreased linearly (p<0.05) as the F:C ratio increased. In contrast with the current results of the goats and Sika deer, the high forage diets generally increased the CH4 production in beef [11] and dairy [9,28] cattle as well as in the modeling [14,15] and batch culture [29] studies. Structural carbohydrate-rich diet causes greater production of enteric CH4 than non-fiber carbohydrate-rich diet in dairy cows [4] because the diet containing large amounts of non-fiber carbohydrates derives propionate production in the rumen, thereby inhibiting rumen methanogen growth [30]. However, some studies for goats showed that the dietary F:C ratio did not affect CH4 (g/d) emission [10,31]. According to the morphophysiological classification of Hofmann [32], goats and Sika deer were intermediate type and concentrate eaters, respectively, whereas cattle and sheep were grass/roughage eaters. As the stomach of concentrate eaters or intermediate types has a lesser capacity, larger opening, faster passage rate, and shorter retention time than grass/roughage eaters [32], more indigested forage contents in the rumino-reticulum pass toward the omasum in concentrate eaters or intermediate types than in grass/roughage eaters. In the current study, the NDF digestibility (NDFD) was similar among the treatments in both goats (p = 0.726) and Sika deer (p = 0.278), whereas the DM digestibility showed a significant difference (p<0.001). For this difference, the low F:C ratio diet may generate more enteric CH4 than the high F:C ratio diet in goats and Sika deer because large amount of energy sources for rumen microbes was available in the low F:C ratio diet than the high F:C ratio diet. It has been widely recognized that the diversity of rumen bacteria community could vary with animal species [33] or geographical region of host animal [34]. As the enteric CH4 production is correlated with rumen microbial community structure [35], these results which comparing to bovine may be explained by rumen bacteria diversity. In addition, the restriction of experimental diets (2% of initial BW) might affect the energy balance of microorganisms in rumen. Interestingly, the results showed that in both goats and deer, the enteric CH4 production expressed as g/kg DDMI and g/kg DOMI was not affected by the F:C ratio. Although several studies [36,37] suggested that the nutrient digestibility did not related to enteric CH4 production, in general, the nutrient digestibility could play an important role in the enteric CH4 production of ruminants [38]. Therefore, digested nutrient intake, which was available for digestion by rumen microorganisms, could be considered when determining the CH4 generation factor in goats and Sika deer.

Effect of forage to concentrate ratio on enteric methane and carbon dioxide in goats (Capra hircus hircus) and Sika deer (Cervus nippon hortulorum)
For goats, equation 1, which used the DMI, NDF concentrate (NDFC), DM digestibility (DMD), and NDFD as independent variables, showed the highest accuracy (Table 4; R2 = 0.85, RMSE = 0.74, and p = 0.059). For Sika deer, equation 5, which used the DMI, NDFC, DMD, and NDFD as independent variables, showed the highest accuracy (R2 = 0.96, RMSE = 0.54, and p = 0.004). For goats, equation 3 that did not use the digestibility factors as variables showed low accuracy (R2 = 0.24, RMSE = 1.38, and p = 0.45), whereas, for Sika deer, equation 7 that did not use digestibility factors as variables showed a relatively high accuracy (R2 = 0.88, RMSE = 0.54, and p = 0.001). The digestibility factors are usually more difficult to measure than the DMI and nutrient concentrate. Therefore, in practice, the model composed without the digestibility is more useful. Although the extant models based on the database organized from different studies on the emission of enteric CH4 by goats have already been developed [16], to our knowledge, the model for the emission of enteric CH4 by deer was not available. Thus, although the models for deer were organized from the limited database, these models will partially help to estimate the enteric CH4 emission from Sika deer.
CONCLUSION
In goats and Sika deer, the F:C ratio decreases the nutrient digestibility and the enteric CH4 emissions expressed as g/d, g/kg BW0.75, % of GEI, g/kg DMI, and g/kg OMI; however, the enteric CH4 emissions expressed as g/kg DDMI and g/kg DOMI were not affected. Therefore, digested nutrient intake, which was available for digestion by rumen microorganisms, could be considered when determining the CH4 generation factor in goats and Sika deer. In addition, as the model for enteric CH4 emission from Sika deer did not exist, the equations that were derived in this study will partially help to estimate the enteric CH4 emission from Sika deer.
ACKNOWLEDGMENTS
This paper was supported by Konkuk University in 2014.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.