 |
 |
Abstract
The present study was carried out to investigate the effects of dietary antioxidants on pro-inflammatory cytokines, heat shock protein (HSP) and antioxidant status in broiler chicks under summer conditions. A total of 162, 3-d-old broiler chicks were randomly assigned to a basal diet (CON) and the basal diet supplemented with vitamin C (200 mg/kg diet, VCD) or vitamin E (100 mg/kg, VED) until 35 day of age. All birds were exposed to summer diurnal heat stress at average daily fluctuations of temperature between 32°C to 34°C at day to 27°C to 29°C at night for the entire feeding periods. There was no significant difference in body weight, feed to gain ratio and the relative organ weight except the thymus in response to dietary vitamin C or E supplementation. However, the mRNA expression of interleukin (IL)-1β, IL-6, interferon (IFN)-γ, Toll like receptor (TLR)-4 and HSP70 in the liver of birds fed diet containing vitamin C significantly (p<0.05) decreased compared with those in birds fed basal diet. Dietary vitamin E also showed a significant (p<0.05) decrease in the mRNA expression of IL-6 and HSP70 compared with a basal diet. Total antioxidant status (TAS) in serum of birds fed vitamin C supplemented diet was significantly (p<0.05) higher with than that in birds a basal diet. Lipid peroxidation in serum and liver resulted in a significant (p<0.05) decrease in response to dietary vitamin C or E supplementation. In conclusion, dietary supplementation with antioxidant vitamins, especially vitamin C resulted in a significant decrease in the mRNA expression of pro-inflammatory cytokines and HSP70, and higher antioxidant parameters than that of birds on the basal diet under summer conditions.
Among various environmental conditions, high ambient temperature beyond the range of the thermoneutral zone in poultry has been known as one of the most fatal stressors, which adversely affects feed intake, growth rate, immunity and mortality (Thaxon and Siegel, 1970; Sahin et al., 2003; Niu et al., 2009). Therefore, preventing and alleviating the heat stress (HS) against summer high ambient temperature is becoming increasingly important in the poultry industry. When chickens are exposed to high temperature, they try to reduce their body temperature within a narrow range through self-thermal regulation to maintain body homeostasis. In particular, the exposure of poultry to summer HS is stressful enough to induce their metabolic rate and physiological responses to cope with the thermal environment (Mckee et al., 1997; Puthpongsiriporn et al., 2001).
In fact, body homeostasis processes are involved in an endogenous cellular defense mechanism that enables cells to cope with stressful HS that induces inflammation and oxidative stress (Molvarec et al., 2011). Although it has been well documented that phagocytosis by macrophages, antibody production and lymphoid organ weighs were significantly decreased by HS in chickens (Thaxon and Siegel, 1970; Bartlett and Smith, 2003; Niu et al., 2009), limited research with chickens has been carried out to investigate whether summer HS could modulate inflammatory cytokines and the antioxidant system. Cytokines, essential proteins of immunity, have been recognized as endogenous signaling molecules that mediate the cellular defense system against inflammatory response induced by high ambient temperature (Hietbrink et al., 2006). In rodent, HS activated the production of pro-inflammatory cytokines such as interleukin (IL)-1β, and IL-6, and tumor necrosis factor (TNF)-α, which ultimately resulted in hemorrhage and necrosis of various organs (Bouchama and Knochel, 2002).
HS can also enormously disturb the balance between the generation of reactive oxygen species (ROS) and the antioxidant system (Tan et al., 2010). The oxidative stress induced by HS causes an increased free radical production, which induces lipid peroxidation and oxidative damage to cellular membranes (Mujahid et al., 2007). Based upon previous study, Bartlett and Smith (2003) described a mechanism by which oxidative stress caused by HS may be interactively associated with the induction of cell-mediated immunity via antioxidant defense system. The extent to which HS induces oxidative stress generally relies on the balance between the production and elimination of oxidants by the antioxidants in chicks. Therefore, scavengers of ROS such as vitamin C and E may play a crucial role in alleviating the cellular damage of tissues and preventing HS during summer. Numerous studies have demonstrated that supplementation of vitamin C or E has a positive effect on maintaining integrity of cellular defense system and subsequent alleviation of HS in chickens under a temperature controlled environment (Mahmoud et al., 2004; Panda et al., 2008). However, limited study has been undertaken to investigate whether dietary antioxidants could modulate pro-inflammatory cytokines and antioxidant status in broiler chicks exposed to summer HS. Therefore, the present study was conducted to evaluate the effects of dietary vitamin C or E on mRNA expressions of pro-inflammatory cytokines and heat shock protein (HSP)-70 and the antioxidant status in broiler chicks under summer conditions.
A total of 162, one-day old broiler chicks (ROSS 308) purchased from a commercial company were maintained in a wire battery cage in a room equipped with forced air ventilation system and a light/dark cycle (light on 04:00–24:00). Immediately after a day acclimation, 3-d-old birds were randomly divided into three groups; each group had six replicate cages with 54 birds. Birds were fed the soy-corn-wheat basal diet (Table 1) supplemented with none (CON), 200 mg/kg of vitamin C (ascorbic acid, purity 99%, VIC) or 100 mg/kg of vitamin E (α-tocopheryl acetate, purity 50%, VIE) until 35 days of age. A thermostat was installed in the room to monitor temperature fluctuation for the entire experimental periods. The weather in summer during experimental period showed a typical East Asian climate, with an average air temperature of 32°C to 34°C during the day and 27°C to 29°C during the night especially at the months of July and August.
All birds received the respective starter (3 to 21 days of age) and finisher (22 to 35 days of age) diets ad libitum and had free access to water nipples. Body weight and feed consumption were monitored on days 3, 21 and 35 to determine growth performance and feed to gain ratio. The animal handling procedures were followed according to the Institutional Animal Care and Use Committee (IACUC) at the Gyeongnam National University of Science and Technology, Korea.
After 35 days of age, six birds with the average body weight per group were sacrificed by cervical dislocation. Immediately after bleeding from the jugular vein, several organs including the liver, spleen and thymus were harvested and weighed. The liver tissue and serum were rapidly frozen in liquid nitrogen and kept at −70°C until further assay.
A quantification of mRNA using Real Time-PCR was carried out to assess mRNA expression of pro-inflammatory cytokines including IL-1β, IL-6, IL-18, interferon (IFN)-γ and toll like receptor (TLR)-4, and HSP70. The cDNA primers to amplify each gene are presented in Table 2. Hepatic tissue from each individual was used for the extraction of total RNA using method of RNAzol B (TRIzol Reagent, Invitron, Eugene, OR, USA). In brief, 2 mL of RNAzol solution was added to 100 mg of hepatic tissues. The tissues were thoroughly homogenized using a glass-glass homogenizer. The lysate was transferred to an Eppendorf-tube and 100 μL of chloroform added and incubated for 5 min on ice. And then, the aqueous phase was obtained by centrifugation for 15 min at 15,000×g. Isolated RNA was precipitated with the same volume of isopropanol and centrifuged for 15 min at 15,000×g. The isolated total RNA was washed with 75% ethyl alcohol, dried, and diluted with diethylpyrocarbonate-treated H2O. The isolated total mRNA was quantified by a spectrophotometer (GeneQuant pro, Amersham, Piscataway, NJ, USA). To synthesize the first strand cDNA, 5.0 μg of total RNA were incubated with 1.0 μg of oligo dT (Invitrogen Co., Carlsbad, CA, USA) at 70°C for 5 min and 4°C for 5 min. The reaction solution was incubated at 42°C for 50 min, 90°C for 10 min and 42°C for 50 min in a reaction mixture containing 5X 1st strand buffer, 2.5 mM dNTP, 0.1 M DTT, superscript III and RT-mixture. After that, 0.5 μL of RNase H was added to RT-products to remove RNA hybridized with cDNA for 20 min at 37°C. The quantification of the target genes and internal control (RPL27) was examined using real-time quantitative PCR with SYBR green supermix (Bio-Rad, Hercules, CA, USA). Real-time PCR assay was performed under the following conditions: 5 min at 95°C, 40 cycles of denaturation at 95°C for 15 s, and annealing at 60°C for 30 s and extension 72°C for 30 s. The PCR amplification cycle at which dye fluorescence passed the selected baseline (Ct) was determined by real-time monitoring and the expression of all mRNAs were normalized by 2[deltadelta] method (Livak and Schmittgen, 2001) to calculate relative changes in gene expression.
Serum total antioxidant status (TAS) was assayed using a Randox reagent set (Randox, Antrim, UK), which was adapted to an enzyme-linked immunosorbent assay (ELISA). The determination is based on the reaction of 2, 29-azino-di-(3-ethylbenzthiazoline sulfonate) (ABTS) with a peroxidase and H2O2 to produce the radical cation ABTS. The radical cation has a relatively stable blue-green color, which can be detected at 600 nm. Antioxidants contained in the sample give rise to the suppression of this color development based on the trolox equivalent antioxidant capacity.
Serum and hepatic microsome were used to analyze lipid peroxidation. To harvest microsomal fractions from the liver, the homogenized tissues were centrifuged at 10,000×g, and the resulting supernatant was centrifuged at 105,000×g in a Centrikon T-2080 (Kontron Instruments, Zürich, Switzerland) ultracentrifuge. The resulting pellet (microsomes) was suspended in a phosphate buffer containing 150 mM KCl (pH 7.4) to produce a protein concentration of 20mg/ml. After then, the microsomes were frozen in liquid nitrogen and kept at −80°C for further assay of lipid perodixation. The lipid peroxidation in serum and microsomes was measured by the amount of 2-thiobarbituric acid (TBA) reactive substances using a spectrophotometer at 532 nm (Bidlack and Tappel, 1973). TBA reaction is expressed as nanomoles of malondialdehyde (MDA) per milligram of protein. Protein was assayed by the BCA method (Thermo Scientific, Rockford, IL, USA) using ELISA plate reader (VMax, Molecular Devices, Sunnyvale, CA, USA).
The effect of the dietary vitamin C or E on data was analyzed by the ANOVA Procedure of SAS (SAS Institute Inc., Cary, NC, USA). When the treatment effect was significant at p<0.05, Tukey test was applied to identify significant differences among dietary groups. The level of probability indicating a statistical difference was determined as p<0.05. Data are presented as means±SD.
Growth performance and the relative organ weights are shown in Table 3 and 4, respectively. Dietary supplementation of vitamin C or E did not affect body weight, feed intake and feed conversion in birds during summer conditions. However, the VIE group was shown to have a significant (p<0.05) increase in the relative thymus weight by 77% compared to the CON group without change in the weights of the liver and spleen. The relative weights of organs did not differ between the VIC and VIE groups.
The mRNA expression levels of pro-inflammatory cytokines (IL-1β, IL-6, IL-18, and IFN-γ) and biomarker proteins (TLR-4 and HSP70) in the liver of birds fed diets supplemented with vitamin C and E are presented in Table 5. The mRNA expression level of IL-1β, IL-6, IFN-γ and TLR-4 in the VIC group was significantly (p<0.05) lower than that in the CON group. Compared with the VIE group, the VIC group resulted in significant (p<0.05) up-regulation of IL-1β, IFN-γ and TLR-4 (Table 5). The VIE group also significantly (p<0.05) increased in mRNA expression of IL-6 compared with the CON group. Furthermore, mRNA expression of HSP70 was markedly (p<0.05) lower in birds fed diet supplemented with vitamin C and E than in those fed a basal diet. Therefore, mRNA expression level of some pro-inflammatory cytokines and HSP70 in response to summer diurnal HS were significantly (p<0.05) attenuated by dietary antioxidant vitamins, particularly vitamin C.
Total antioxidant status (TAS) and lipid peroxidation (MDA) in birds fed diets supplemented with vitamin C and E during summer HS are shown in Figure 1 and 2, respectively. TAS in serum was significantly (p<0.05) higher in the VIC group compared with the CON group (Figure 1). Moreover, dietary supplementation with vitamin C or E to birds subjected to summer HS markedly (p<0.05) decreased lipid peroxidation (MDA) in serum or liver compared with the CON group (Figure 2). There was no difference in TAS and MDA between the VIC and VIE groups.
In the present study, growth performance, feed intake and feed to gain ratio were not affected by dietary supplementation of vitamin C and E when birds are exposed to high ambient temperature under summer conditions. This observation was in agreement with previous studies (Bartov and Frigg, 1992, 1993: Mckee et al., 1997), whereas Niu et al. (2009) reported that dietary vitamin E improved feed conversion without affecting body weight (BW) and feed intake in birds under HS. By contrast, Sahin et al (2003) reported that quails fed vitamin C supplemented diet under HS improved feed intake and feed efficiency compared with heat stressed quail without affecting BW. In this study, the relative weight of thymus was higher in birds fed diet supplemented with vitamin E than that in the control birds without affecting other organ weights. Niu et al. (2009) reported that weights of all lymphoid organs including thymus, bursa and spleen were not affected by dietary vitamin E, although all organ weights in birds exposed to HS was significantly decreased compared with thermo-neutral birds. From our study, it is assumed that increased thymus weight in birds fed diet supplemented with vitamin E may have a beneficial effect on improving immunity under stressed conditions, although growth performance was not affected in summer HS conditions.
To investigate the effects of dietary vitamin C or E on immune response in birds exposed to summer HS, we analyzed the mRNA expression of pro-inflammatory indicators such as IL-1β, IL-6, IL-18, IFN-γ, and TLR-4 in the liver. In this study, birds fed diet supplemented with vitamin C during summer conditions showed a significant down-regulation of hepatic IL-1β, IL-6, IFN-γ, and TLR-4 compared with those fed basal diet or supplemented with vitamin E. Dietary vitamin E also significantly suppressed the mRNA expression of IL-6 compared with the CON group. It has been demonstrated that antioxidant vitamins activated immune function through increasing the proliferation of lymphocytes and macrophages in chickens under high ambient temperature (Puthpongsiriporn et al., 2001; Niu et al., 2009). In particular, IL-1β and IL-18, which belong to the IL-1 family of pro-inflammatory cytokines, are actively involved in an inflammatory response and the secretion of antibody under hyperthermia in animal models (Lin et al., 1994; Helwig and Leon, 2011). Similar to our observation, it has been reported that dietary supplementation of antioxidant vitamins decreased the expression of pro-inflammatory cytokines such as IL-6, IL-2 and TNF-α in rodents (Amarakoon et al., 1995; Yun et al., 2012). Yun et al. (2012) also reported that in heat stressed rats dietary vitamin C supplementation produced significantly lower mRNA levels of hepatic IL-6 and TNF-α than controls. A study with chickens under tropical summer HS has demonstrated that vitamin C and E could be applied to alleviate the negative effects on growth performance and immunity in response to HS (Panda et al., 2008).
Since dietary antioxidant vitamins are known to have a beneficial effect on alleviating HS, we analyzed the mRNA expression of HSP70. Our observation is in accordance with the results of previous studies that HSP70 expression in vitamin C and E-fed birds was much lower than that in control birds. Mahmoud et al. (2004) reported that chickens fed a diet supplemented with vitamin C showed a significantly lower HSP70 expression compared with control chickens. It is well documented that HS activates the expression of HSPs, which are a set of conserved proteins induced by severe HS. HSPs induced by high ambient temperature mediate the cellular defense mechanism through the activation of nuclear factor (NF)-kB, which induces the expression of pro-inflammatory cytokines (Pockley, 2003). Therefore, summer HS could induce the expression of HSP70 and subsequently activate the expression of several pro-inflammatory cytokines (Welc et al., 2012; Yun et al, 2012). Several studies also reported that down-regulation of HSP expression in response to dietary antioxidant vitamins under HS might be associated with the modulation of pro-inflammatory cytokine expression (Chang et al., 2010; Khassaf, et al., 2003; Rettenbacher and Palme, 2009), suggesting that dietary antioxidant may alleviate HS in birds during summer conditions.
However, previous studies gave some conflicting results that the dietary antioxidants could selectively affect the inhibition of pro-inflammatory expression to different extents depending upon type of stressor, genetic characteristics of bird, and level and sort of dietary antioxidant vitamins (Schwager and Schulze, 1998; Jacob et al., 2008; Redmond et al., 2010). Under circumstance of summer HS in this study, dietary supplementation of vitamin C exerted a greater positive effect on the suppression of pro-inflammatory cytokines than dietary vitamin E. It appears that vitamin E has significantly higher immunomodulatory effects when chickens are infected with bacteria (Leshchinskey and Klasing, 2003). Kaiser et al. (2012) reported that vitamin E supplementation alleviated the expression of lipopolysaccharide (LPS)-induced pro-inflammatory cytokines such as IL-6 in chickens.
Based upon previous studies, there is substantial evidence that a mechanism by which the increased expression of pro-inflammatory cytokines induced by HS might be interactively associated with the generation of ROS (Lin et al., 2006; Tan et al., 2010). As a consequence, HS can significantly interrupt the balance between the production of ROS and the antioxidant system in birds reared under chronic HS (Lin et al., 2008). The up-regulation of pro-inflammatory cytokines caused by HS can profoundly produce oxidative stress, since enhanced immune function produces excessive ROS (Chang et al., 2010; Yun et al., 2012). Moreover, the enhanced production of ROS during HS can induce remarkable damage to cellular membranes by disturbing the antioxidant defense system (Omar and Pappolla, 1993). Several studies indicated that the increased ROS during the exposure to HS resulted in a significant impact on lipid peroxidation (Young, 1990; Mujahid et al., 2007; Lin et al., 2008).
Numerous studies have previously demonstrated that dietary antioxidant vitamins have a scavenging effect on reducing oxidative stress by the elimination of excessive ROS (Halliwell and Gutteridge, 1989; Yu, 1994), leading to a reduction of oxidative stress indicators in birds (Schwager and Schulze, 1998; Puthpongsiriporn et al., 2001). Thus, dietary vitamin C and E may be responsible for modulating pro-inflammatory cytokine expression via antioxidant defense system in birds exposed to summer HS.
Thereafter, we examined TAS and lipid peroxidation in birds raised during summer conditions. In the present study, dietary vitamin C significantly increased serum TAS and decreased serum and hepatic lipid perioxidation. Dietary supplementation of vitamin E to birds also decreased hepatic lipid peroxidation. The fact that there was no obvious change in serum TAS of birds fed diet supplemented with vitamin E was presumably due to the nature of vitamin E. Vitamin E is an excellent chain breaking antioxidant that exists and protects membranes from oxidative damage (Yu, 1994). Hence, it is assumed that the serum TAS in vitamin E fed birds might not be affected by vitamin E, since this vitamin is naturally present in cellular membrane. In particular, several studies demonstrated that dietary vitamin C and E showed a significant decrease in MDA value, as an indicator of lipid peroxidation (Cherian et al., 1996; Sahin et al., 2003). Therefore, both dietary vitamins may positively function in preventing lipid peroxidation in birds exposed to summer HS, indicating that there is a strong functional association between pro-inflammatory cytokines and the antioxidant system in birds. Dietary antioxidants exert their positive effects on the elimination of ROS and subsequently prevent the activation of NF-kB-mediated inflammation process (Conner and Grisham, 1996). Therefore, dietary antioxidant vitamins suppressed the mRNA expression of pro-inflammatory cytokines and HSP70 via stabilizing antioxidant status in birds under summer conditions.
In conclusion, dietary supplementation of vitamin C and E to bird exposed to summer HS showed alleviating effects on heat stress-induced inflammation and oxidative stress. This study also suggests that vitamin C may be more effective for alleviating summer HS than vitamin E in birds, although both antioxidant vitamins exerted a beneficial effect on maintaining immunity and antioxidant status under summer conditions.
ACKNOWLEDGMENTS
This research was supported by a grant from the Next-Generation Biogreen 21 Program (No. PJ007981), Rural Development Administration, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea. This project was also partially supported by the Gyeongnam National University of Science and Technology (2013).
Figure 1
Total antioxidant status (TAS) in serum from 35 day-old broiler chicks fed the diets the basal diet (CON) and the diets containing vitamin C (VIC) and vitamin E under summer conditions. a,b Values (mean±SD, n = 6) with different superscripts are significantly (p<0.05) different among treatment.
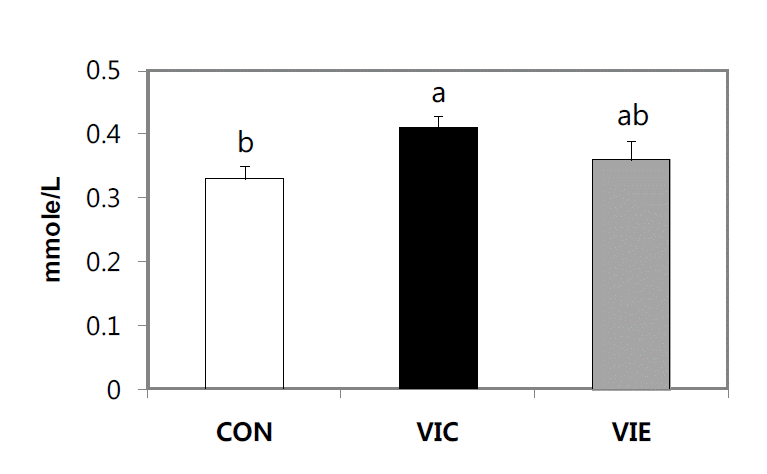
Figure 2
Lipid peroxidation in serum (A) and the liver (B) from 35 day-old broiler chicks fed the diets the basal diet (CON) and the diets containing vitamin C (VIC) and vitamin E under summer conditions. a,b Values (mean±SD, n = 6) with different superscripts are significantly (p<0.05) different among treatment.
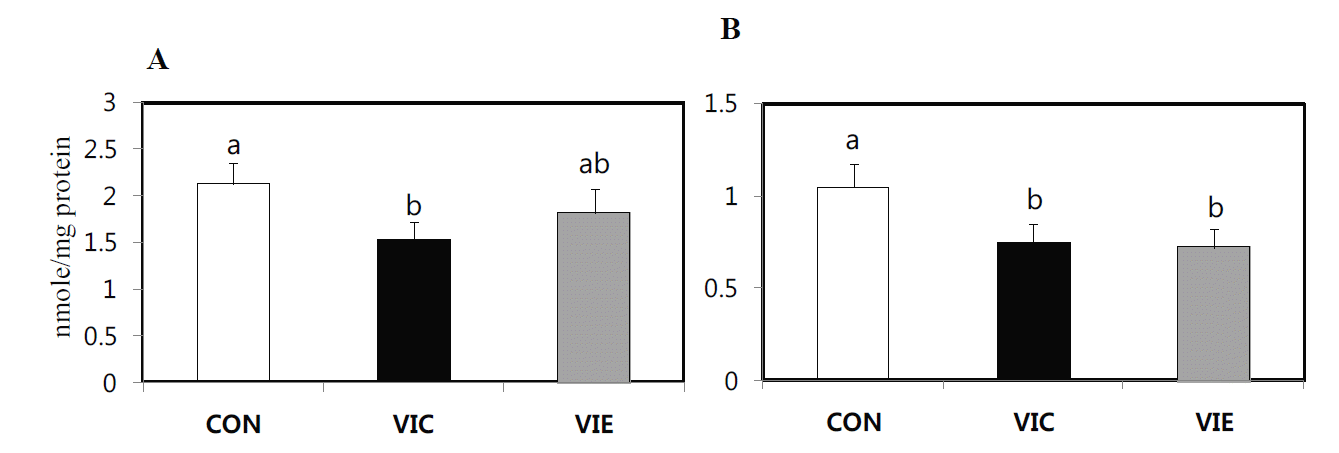
Table 1
Formula of the basal diets fed to broiler chicks
| Item | Basal diets | |
|---|---|---|
|
|
||
| Starter | Finisher | |
| Ingredients (%) | ||
| Corn | 38.26 | 44.28 |
| Wheat | 20.00 | 20.00 |
| Wheat bran | 5.00 | 4.00 |
| Animal fat | 2.20 | 3.00 |
| Corn gluten | 4.00 | 4.00 |
| Soybean meal (44% CP) | 23.00 | 16.50 |
| Rapeseed meal | 1.50 | 2.00 |
| Fish meal | 1.00 | 1.00 |
| Meat meal | 2.00 | 2.00 |
| Salt | 0.20 | 0.23 |
| Calcium carbonate | 0.40 | 0.20 |
| Tricalcium phosphate | 1.40 | 1.60 |
| Lysine (liquid) | 0.46 | 0.66 |
| Methionine | 0.13 | 0.12 |
| Choline-HCl | - | 0.01 |
| Vitamin premix1 | 0.20 | 0.20 |
| Mineral premix2 | 0.20 | 0.20 |
| Maduramicin+nicarbazine | 0.05 | |
| Chemical composition (%) | ||
| Crude protein | 21.1 | 19.3 |
| Ether extract | 4.65 | 5.10 |
| Crude fiber | 4.10 | 3.80 |
| Crude ash | 4.95 | 4.85 |
Table 2
Primers used for the quantification of mRNA by real time PCR
Table 3
Growth performance, feed intake and feed to gain ratio from 35 day-old broiler chicks fed the basal diet (CON) and the diets containing vitamin C (VIC) and vitamin E (VIE) under summer conditions
Table 4
The relative weights of immune related organs from 35 day-old broiler chicks fed the diets the basal diet (CON) and the diets containing vitamin C (VIC) and vitamin E (VIE) under summer conditions
| Item | Dietary treatment | ||
|---|---|---|---|
|
|
|||
| CON | VIC | VIE | |
| ———g/100 g BW ——— | |||
| Liver | 2.32±0.28 | 2.22±0.43 | 2.11±0.17 |
| Spleen (g) | 0.11±0. 04 | 0.10±0.05 | 0.10±0.03 |
| Thymus (g) | 0.13±0. 04b | 0.19±0.04ab | 0.23±0.07a |
| Bursa of Fabricius (g) | 0.09±0. 03 | 0.12±0.08 | 0.13±0.07 |
Table 5
mRNA expression of pro-inflammatory cytokines and heat shock protein 70 (HSP70) in the liver from 35 day-old broiler chicks fed the diets the basal diet (CON) and the diets containing vitamin C (VIC), and vitamin E under summer conditions
| Cytokines | Dietary treatment | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| CON | VIC | VIE | ||||
|
|
|
|
||||
| ΔCt | 2−ΔΔCt | ΔCt | 2−ΔΔCt | ΔCt | 2−ΔΔCt | |
| IL-1β | 7.23±0.44b | 1.00 | 9.00±0.29a | 0.29 | 7.43±1.45b | 0.71 |
| IL-6 | 10.68±1.59b | 1.00 | 14.42±1.92a | 0.07 | 12.98±1.52a | 0.20 |
| IL-18 | 7.45±0.39 | 1.00 | 8.60±0.35 | 0.45 | 8.23±1.14 | 0.56 |
| IFN-γ | 9.61±0.60b | 1.00 | 13.03±1.32a | 0.09 | 9.17±0.95b | 1.35 |
| TLR-4 | 8.73±0.78b | 1.00 | 10.75±1.17a | 0.25 | 9.17±1.32b | 0.72 |
| HSP70 | 5.13±0.57c | 1.00 | 8.76±1.20a | 0.08 | 6.72±0.44b | 0.33 |
REFERENCES
Amarakoon AM, Tappia PS, Grimble RF. 1995. Endotoxin induced production of interleukin-6 is enhanced by vitamin E deficiency and reduced by black tea extract. Inflamm Res 44:301–305.


Bartlett JR, Smith MO. 2003. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult Sci 82:1580–1588.


Bartov I, Frigg M. 1992. Effect of high concentrations of dietary vitamin E during various age periods on performance, plasma vitamin E and meat stability of broiler chicks at 7 weeks of age. Br Poult Sci 33:393–402.


Chang WH, Hu SP, Huang YF, Yeh TS, Liu JF. 2010. Effect of purple sweet potato leaves consumption on exercise-induced oxidative stress and IL-6 and HSP72 levels. J Appl Physiol 109:1710–1715.


Cherian G, Wolfe FW, Sims JS. 1996. Dietary oils with added tocopherols: Effects on egg or tissue tocopherols, fatty acids, and oxidative stability. Poult Sci 75:423–431.


Halliwell B, Gutteridge JMC. 1989. Free Radicals in Biology and Medicine. Clarendon Press; Oxford, UK:
Helwig BG, Leon L. 2011. Tissue and circulating expression of IL-1 family members following heat stroke. Physiol Genomics 43:1096–1104.


Hietbrink F, Koenderman L, Rijkers GT, Leenen LPH. 2006. Trauma: The role of the innate immune system. World J Emerg Surg 1:15



Jacob K, Periago MJ, Böhm V, Berruezo GR. 2008. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br J Nutr 99:137–146.


Kaiser MG, Block SS, Ciraci C, Fang W, Sifri M, Lamont SJ. 2012. Effects of dietary vitamin E type and level on lipopolysaccharide-induced cytokine mRNA expression in broiler chicks. Poult Sci 91:81893–1898.


Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ. 2003. Effect of vitamin C supplements on antioxidant defense and stress proteins in human lymphocytes and skeletal muscle. J Physiol 549:645–652.



Leshchinsky TV, Klasing KC. 2003. Profile of chicken cytokines induced by lipopolysaccharide is modulated by dietary alpha-tocopheryl acetate. Poult Sci 82:1266–1273.


Lin MT, Kao TY, Su CF, Hsu SS. 1994. Interleukin-1 beta production during the onset of heat stroke in rabbits. Neurosci Lett 174:17–20.


Lin H, De Vos D, Decuypere E, Buyse J. 2008. Dynamic changes in parameters of redox balance after mild heat stress in aged laying hens (Gallus gallus domesticus). Comp Biochem Physiol Part C Toxicol Pharmacol 147:30–35.

Lin H, Decuypere E, Buyse J. 2006. Acute heat stress induces oxidative stress in broiler chickens. Comp Biochem Physiol Part A Mol Integr Physiol 144:11–17.

Livak KJ, Schmittgen TD. 2001. Analysis is of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408.


Mahmoud KZ, Edens FW, Eisen EJ, Havenstein GB. 2004. Ascorbic acid decreases heat shock protein 70 and plasma corticosterone response in broilers (Gallus gallus domesticus) subjected to cyclic heat stress. Comp Biochem Physiol Part B Biochem Mol Biol 137:35–42.

McKee JS, Harrison PC, Riskowski GL. 1997. Effects of supplemental ascorbic acid on the energy conversion of broiler chicks during heat stress and feed withdrawal. Poult Sci 76:1278–1286.


Molvarec A, Szarka A, Walentin S, Beko G, Karádi I, Prohászka Z, Rigó T. 2011. Serum heat shock protein 70 levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in women with preeclampsia. Clin. Chim Acta 412:1957–1962.


Mujahid A, Akiba Y, Warden CH, Toyomizu M. 2007. Sequential changes in superoxide production, anion carriers and substrate oxidation in skeletal muscle mitochondria of heat-stressed chickens. FEBS Lett 581:3461–3467.


Niu ZY, Liu FZ, Yan QL, Li WC. 2009. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult Sci 88:2101–2107.


Omar R, Pappolla M. 1993. Oxygen free radicals as inducers of heat shock protein synthesis in cultured human neuroblastoma cells: Relevance to neurodegenerative disease. Eur Arch Psychiatry Clin Neurosci 242:262–267.


Panda AK, Ramarao SV, Raju MV, Chatterjee RN. 2008. Effect of dietary supplementation with vitamins E and C on production performance, immune responses and antioxidant status of White Leghorn layers under tropical summer conditions. Br Poult Sci 49:592–599.


Puthpongsiriporn U, Scheideler SE, Sell JL, Beck MM. 2001. Effects of vitamin E and C supplementation on performance, in vitro lymphocyte proliferation, and antioxidant status of laying hens during heat stress. Poult Sci 80:1190–1200.


Redmond SB, Tell RM, Coble D, Mueller C, Palic D, Andreasen CB, Lamont SJ. 2010. Differential splenic cytokine responses to dietary immune modulation by diverse chicken lines. Poult Sci 89:1635–1641.


Rettenbacher S, Palme R. 2009. Biological validation of a non-invasive method for stress assessment in chickens. Berl Munch Tierarztl Wochenschr 122:8–12.

Sahin K, Onderci M, Sahin N, Gursu MF, Kucuk O. 2003. Dietary vitamin C and folic acid supplementation ameliorates the detrimental effects of heat stress in Japanese quail. J Nutr 133:1882–1886.


Schwager J, Schulze J. 1998. Modulation of interleukin production by ascorbic acid. Vet Immunol Immunopathol 64:45–57.


Tan GY, Yang L, Fu YQ, Feng JH, Zhang MH. 2010. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult Sci 89:115–122.


Thaxon P, Siegal HS. 1970. Immunodepression in young chickens by high environment temperature. Poult Sci 49:202–205.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





