 |
 |
Abstract
The glucagon-like peptide 2 (GLP-2) that is expressed in intestine epithelial cells of mammals, is important for intestinal barrier function and regulation of tight junction (TJ) proteins. However, there is little known about the intracellular mechanisms of GLP-2 in the regulation of TJ proteins in pigletsŌĆÖ intestinal epithelial cells. The purpose of this study is to test the hypothesis that GLP-2 regulates the expressions of TJ proteins in the mitogen-activated protein kinase (MAPK) signaling pathway in pigletsŌĆÖ intestinal epithelial cells. The jejunal tissues were cultured in a DulbeccoŌĆÖs modified EagleŌĆÖs medium/high glucose medium containing supplemental 0 to 100 nmol/L GLP-2. At 72 h after the treatment with the appropriate concentrations of GLP-2, the mRNA and protein expressions of zonula occludens-1 (ZO-1), occludin and claudin-1 were increased (p<0.05). U0126, an MAPK kinase inhibitor, prevented the mRNA and protein expressions of ZO-1, occludin, claudin-1 increase induced by GLP-2 (p<0.05). In conclusion, these results indicated that GLP-2 could improve the expression of TJ proteins in weaned pigsŌĆÖ jejunal epithelium, and the underlying mechanism may due to the MAPK signaling pathway.
Tight junction (TJ) is an important mode of cell-to-cell adhesions in the epithelial and endothelial cellular sheets, which act as a primary barrier that prevents the diffusion of solutes through the intercellular space, especially in the intestine (Tsukita et al., 2006). Weaned pigs are exposed to various stresses (e.g. the changes of feed, environment and ablactation) and infections caused by enterotoxigenic Escherichia coli (Osek, 1999). These factors reduce the expression of intestinal TJ proteins and increase the epithelial permeability of intestines (Bruewer et al., 2003). The glucagon-like peptide 2 (GLP-2), an intestinotrophic growth hormone, in recent years has been proved to have the biological function of rapidly improving the intestinal epithelial barrier. It could significantly reduce intestinal fluxes of Na+, Cr-EDTA (Cameron et al., 2005), horseradish peroxidase (HRP) (Benjamin et al., 2000; Cameron et al., 2005), and bacterial penetration and colonic inflammatory cells (Cameron et al., 2005). Under stress conditions, GLP-2 effectively enhances the barrier function and reduces the amount of luminal antigen that penetrates the lamina propria (Cameron et al., 2003). Cameron et al. (2005) thought these effects were due to changes in the TJ proteins induced by GLP-2. GLP-2 could recover and maintain the integrity of the intestinal mucosa epithelium barrier by increasing the expressions of zonula occludens-1 (ZO-1), claudin-1 and occludin in obstructive jaundice rats (Chen et al., 2008). GLP-2 could modulate the intestinal cellsŌĆÖ proliferation and apoptosis via the ERK1/2 dependent mechanism (Burrin et al., 2001). However, there are no studies of GLP-2 regulating the expression of TJ proteins in the weaned pigs jejunal epithelium. Therefore, the present experiments were conducted to determine the effect of GLP-2 on intestinal tight junction in weaned pigs and to test the hypothesis that mitogen-activated protein kinase (MAPK) signaling pathway plays an important role in GLP-2-mediated modulation of the TJ proteins.
The protocol of the present experiment was approved by the Animal Care and Use Committee, Sichuan Agricultural University, YaŌĆÖan, China.
Three weaned DLY (Duroc├ŚLandrance├ŚYorkshire) pigs (28 d of age) were starved for 12 h (the average body weight was 7.28┬▒0.32 kg). At that point, they were anaesthetized by the intravenous injection of chlorpromazine and subsequently killed by exsanguination. The abdomen was immediately opened and the jejunum was excised and dissected into small segments (5 to 7 cm). The tissue segments were carefully rinsed in sterile PBS containing penicillin (200 IU/mL) and streptomycin (200 ╬╝g/mL) at 37┬░C. Under sterile conditions, the jejunal tissue was dissected into 5 mm pieces in DulbeccoŌĆÖs modified EagleŌĆÖs medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco), insulin (5 ╬╝g/mL), transferrin (5 ╬╝g/mL), penicillin (100 IU/mL) and streptomycin (100 ╬╝g/mL) for 24 h. Then, two pieces of tissue were cultured in a single well which was pipetted with 1.5 mL of fresh culture medium with different concentrations of GLP-2 (PurityŌēź95%, Phoenix pharmaceuticals, USA). The concentrations of GLP-2 were 0, 10, and 100 nmol/L respectively. Also, 48 pieces of tissue were cultured for each individual level of medium. Tissues were maintained in 24 well culture plates (Corning) at 37┬░C in a humidified incubator in an atmosphere of 5% CO2. The medium was replaced every 24 h.
After being cultured for 72 h, the tissue pieces were collected so that the effects of GLP-2 treatment on the TJ proteins and their mRNA expressions could be analyzed. Once the optimum dose of GLP-2 that could improve the protein expression of TJ proteins was established, another three DLY weaned piglets (28 d of age) were slaughtered (the average body weight was 7.65┬▒0.58 kg). The jejunal tissues were collected under sterile conditions and random pieces were transferred to a fresh medium in 24 well tissue culture plates. Further, two pieces of tissues were cultured in a single well in 1.5 mL of the culture medium. The culture medium contained: a control group, a 100 nmol/L GLP-2-treated group and a 100 nmol/L GLP-2 with 10 ╬╝mol/L U0126 (Calbiochem-Novabiochem Corp, in San Diego, CA) -treated group. 48 pieces of tissues were cultured for each individual level of medium. Tissues were maintained in 24 well culture plates (Corning) at 37┬░C in a humidified incubator in an atmosphere of 5% CO2. The medium was replaced every 24 h. After being cultured for 72 h, the jejunal tissues were collected and quickly frozen in liquid nitrogen and stored at ŌłÆ80┬░C or to be used in subsequent trials.
TRIZOL reagent (TaKaRa) was added to samples of jejunal tissues. RNA extraction was performed using the chloroform/isopropyl alcohol method, followed by Qiagen RNeasy clean-up, according to the manufacturerŌĆÖs instructions. RNA was eluted from the column in 50 ╬╝L of RNase-free water, and concentration and purity determined using a DU-800 spectrophotometer (Beckman, Brea, CA, USA). Reverse transcription PCR was performed using the PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time) (Code No. RR047A; TaKaRa, China). Reaction conditions and PCR program followed the manufacturerŌĆÖs instructions. Different porcine gene specific primers (Invitrogen, Shanghai, China) were used (Table 1). Primer pairs were designed using Primer3 program based on NCBI porcine sequences.
Real time PCR (RT-PCR) was performed using a CFX-96 real time PCR system (Bio-Rad, California, US) with the SYBR Premix Ex Taq TM II (Tli RNaseH Plus) (Code No. RR820A; TaKaRa, China). cDNA (1 ╬╝L) was added in a 25 ╬╝L reaction mixture which contained 12.5 ╬╝L SYBR Premix Ex Taq II (Tli RNaseH Plus) (2├Ś), 10 ╬╝M each primer, 2 ╬╝L cDNA and 8.5 ╬╝L dH2O. The program setting was 30 s at 95┬░C and then 40 cycles of 95┬░C for 5 s, 53┬░C to 62┬░C (depending on the primers used) for 30 s, and 72┬░C for 30 s. On the completion of amplification, a melting curve was produced by holding the reaction mixture at 60┬░C to 95┬░C with a heating rate of 0.1┬░C per second and a continuous fluorescence measurement and finally a cooling step to 40┬░C. The housekeeping gene encoding ╬▓-actin was used as an internal control. The analysis consisted of at least five independent experiments. Each experimental sample was assayed using three replicates for each primer. The Relative levels of the mRNA of the genes of interest were normalized to that of the ╬▓-actin mRNA and were determined using a modified 2ŌłÆ╬ö╬öCt method (Pfaffl et al., 2001).
Western blotting was performed by the modified method of Kansagra et al. (2003). To summarize, 100 mg of pulverised frozen tissue was homogenised in 1 mL of lysis buffer containing 50 mM-2-amino-2-hydroxymethyl-propane-1, 3-diol (Tris)-HCl, 100 mM-phenylmethylsulfonyl fluoride, 0.5 mM-sodium dodecylsulfate and 1 mM-dithiothreitol. After the protein concentration of supernatant fractions was quantized by a standard bicinchoninic acid (BCA) protein assay (Bio-Rad, USA), equal protein amounts (60 to 100 ╬╝g) were separated on 6% to 15% denatured SDS-PAGE gels and run at 100 V for 60 min. Separated proteins were then transferred onto PVDF membranes (Bio-Rad, USA) at 90 V for 45 to 60 min. The membranes were blocked with blocking buffer (concentrated saline and Hammersten casein solution) at room temperature for 1 h and probed with primary antibody overnight. The primary antibodies used were: polyclonal anti-claudin-1 (Santa Cruz Biotechnology Inc., California, USA) diluted 1:500, anti-occludin (Santa Cruz Biotechnology Inc., USA) diluted 1:450, anti-ZO-1 (Santa Cruz Biotechnology Inc., USA) diluted 1:400 and anti-╬▓-actin (Cell Signaling Technology Inc., Massachusetts, USA) diluted 1:2,000. After being washed extensively to eliminate nonspecific binding, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (anti-rabbit IgG) (Santa-Cruz Biotechnology Inc., US) at room temperature for 30 min. The antibody-reactive bands were visualized using chemiluminescence.
The phosphorylated forms of specific signaling proteins in jejunal tissue were measured. Frozen jejunal tissue mucosa samples (100 mg) were homogenized in 50 mM HEPES buffer (pH 7.4) containing 1 mM EDTA, 1 mM dithiothreitol, 5 mg/L phenylmethylsulfonyl fluoride, 5 mg/L aprotinin, 5 mg/L chymostatin, and 5 mg/L pepstatin added phosphatase inhibitor, sodium orthovanadate to a final concentration of 2 mM. The homogenate was then sonicated and centrifuged at 12,000 g for 15 min at 4┬░C. Equal amounts (60 to 100 ╬╝g) of supernatant protein extracts were separated on a 9% to 12% denatured SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with blocking buffer (concentrated saline and Hammersten casein solution) at room temperature for 1 h and probed with primary antibody overnight. The primary antibodies used were: polyclonal anti-p-ERK1/2 (Thr202/Tyr204) (Santa Cruz Biotechnology Inc., USA) diluted 1:1,000, anti-p-p90RSK (Ser380) (Santa Cruz Biotechnology Inc., USA) diluted 1:2000 and then the other manipulation and secondary antibody was administered as in the same way as noted above.
Immunohistochemistry was done to assess the distribution of the TJ proteins ZO-1, claudin-1 and occludin. Every 3 jejunal tissue samples from each group were fixed in fresh neutral buffered 4% paraformaldehyde overnight. Samples were embedded in paraffin blocks, sectioned (5 ╬╝m), and mounted on glass slides. All of sections were dewaxed in xylene and rehydrated and then boiled in 0.1 mol/L sodium citrate buffers (pH 6.3) for antigen retrieval. mouse anti-ZO-1 (1:100; Cell Signaling Technology Inc., USA), mouse anti-claudin-1 (1:100; Cell Signaling Technology Inc., USA) and mouse anti-cccludin (1:150; Cell Signaling Technology Inc., USA) antibodies were applied to assess protein distribution in the tissues. The primary antibodies were used for about 12 h at 4┬░C. The biotinylated goat anti-mouse IgG was used as the secondary antibody. The immunoreactivity was visualized by the avidin-peroxidase method with no specific staining negative control experiments. The OD value of each sample was determined for the quantity access at a 400 magnification with a Carl Zeiss A200 microscope (Carl Zeiss, Germany), additional Image-Pro Plus 6.0 software.
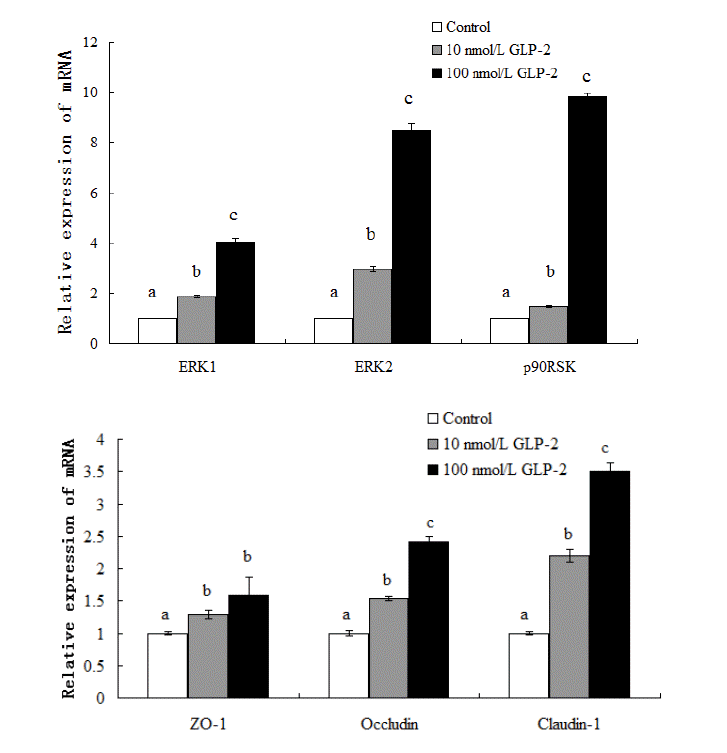
The results showed that supplementing with GLP-2 significantly increased (p<0.05) the expression of ERK1/2 and p90RSK mRNA in the jejunal mucosa (Figure 1). Compared with the control, the expressions of ERK1, ERK2, p90RSK, ZO-1, claudin-1, occludin mRNA increased 85%, 195%, 47%, 29%, 120%, and 54% respectively in the 10 nmol/L GLP-2-treated group and 301%, 747%, 883%, 59%, 251%, and 142% respectively in the 100 nmol/L GLP-2-treated group while the abundance of total ERK1/2 protein was unchanged (p>0.05) (Figure 3).
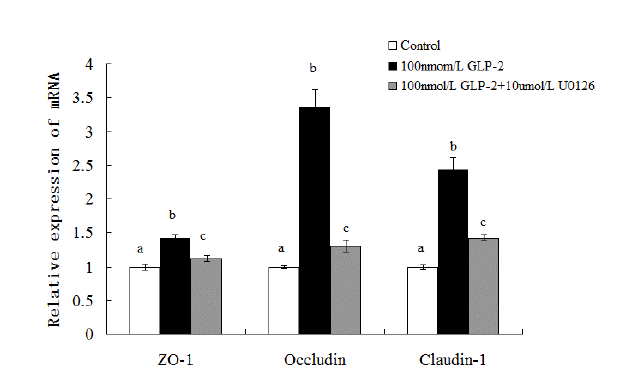
As depicted in Figure 2, compared with the control, the addition of GLP-2 (100 nmol/L) could increase the expressions of ZO-1, claudin and occludin mRNA (p<0.05) while these effects were blocked when U0126 (10 ╬╝mol/L) was simultaneously added into the medium with GLP-2. U0126 inhibited GLP-2-induced up regulation of ZO-1, claudin and occludin mRNA expressions (p<0.05). Compared with the 1├Ś10ŌłÆ7 mol/L GLP-2-treated group, the mRNA expressions of ZO-1, claudin and occludin decreased 21.1%, 61.3%, and 41.6% respectively in the 100 nmol/L GLP-2 with 10 ╬╝mol/L U0126-treated group (p<0.05).
p90RSK is a downstream nuclear target of ERK1/2 and its expression increased after 72 h of GLP-2 treatment based on protein abundance. Both phospho-ERK1/2 and phospho-p90RSK abundance increased significantly (p<0.05). In comparison with the control group, the protein relative abundance of p-ERK1/2, p-p90RSK, ZO-1, claudin-1 and occludin increased 34.7%, 56%, 35.6%, 30.4%, and 26.2% respectively (p<0.05) in the 10 nmol/L GLP-2-treated group; the relative abundance increased 69.5%, 84%, 46.7%, 56.7%, and 35.6% respectively (p<0.05) in the 100 nmol/L GLP-2-treated group (Figure 3).
As shown in Figure 4, in comparison with the control group, GLP-2 (100 nmol/L) increased the abundance of all protein (p<0.05). But when U0126 (10 ╬╝mol/L) was added into the medium, the relative abundance of p-ERK1/2, p-p90RSK, ZO-1, claudin-1, and occludin significantly decreased 14.4%, 20.9%, 17.2%, 24.6%, and 12.6% respectively (p<0.05).
In comparison with the control group, GLP-2 (100 nmol/L) influenced the distribution of ZO-1, occludin and claudin-1 in the tissues. The results show that the distribution of ZO-1 proteins in the GLP-2 groups presented a honeycomb appearance along the enterocyte membranes on the villus top with a more intact structure than in the control group. The occludin and claudin-1 proteins were characterized by a particle-shaped linear distribution along the intestinal villus and were more uniform than in the control group. But when U0126 (10 ╬╝mol/L) was added into the medium, the ZO-1, occludin and claudin-1 proteins presented as a vague and incoherent shape in the villus compared to the 100 nmol/L GLP-2-treated group (Figure 5).
The experimental results indicated that GLP-2 (100 nmol/L) increased the optical density of ZO-1, occludin and claudin-1 positive staining by 18.6%, 40.6%, and 27.6% respectively (p<0.05); while when U0126 (10 ╬╝mol/L) was added into the medium, the distribution of ZO-1, occludin and claudin-1 were sparse in the tissue and the optical density of ZO-1, occludin and claudin-1 positive staining decreased by 9.80%, 28.6%, and 16.2% (p<0.05) compared with the 100 nmol/L GLP-2-treated group (Figure 5).
As a physiological regulator of mucosal epithelial homeostasis, GLP-2 plays an important role in intestinal health. The available evidence suggests that GLP-2 has the function of regulating the mucosal integrity, permeability and nutrient absorption. GLP-2 may potentially exert therapeutically favorable effects on diseases induced by injury or dysfunction of the gastrointestinal epithelium (Drucker et al., 2002). Drucker et al. (1999) found that GLP-2 had the biological function of attenuating small bowel shortening, enhancing the mucosal area and integrity in animal models experiencing inflammatory bowel disease and intestinal injury. These effects may be due to an increase of the mucosal DNA content and proteins by GLP-2 (Kato et al., 1999; Prasad et al., 2000). The GLP-2 receptor, which expresses in a highly tissue-specific manner, distinctly mediates the functions of GLP-2 in the gut and predominantly expresses in the jejunum (Munroe et al., 1999). GLP-2 also has the function of improving the barrier primarily regulated by TJ (Torsten et al., 2001).
Tight Junction is an important junctional complex of simple epithelial cells that is located at the most-apical part of the lateral membrane and contains several unique proteins, such as claudins, the transmembrane protein occludin and ZOs. ZO-1, occludin and claudin-1 are the most important and critical components in the structural and functional organization of the TJ. Occludin is identified as the first integral membrane protein localized at TJ and has important functions in maintaining the integrity and barrier function of the TJ (Furuse et al., 1993). ZO-1 is an important linker protein in TJ. Its PDZ domains could bind to C-terminal sequences of claudins and its GUK domains could bind to the C-terminal tail of occludin. ZO-1 also constitutes a link between the plasma membrane and actin cytoskeleton to make the complex stable (Fanning et al., 1998). Claudin-1 was fairly tightly related to the expression of ZO-1 in the small intestine. As claudin-1 has two extra cellular loops that display variability in the distribution and number of charged residues, it is the major determinant of ionic selectivity in paracellular transport processes (Gonzalez et al., 2008).
Benjamin et al. (2000) demonstrated that GLP-2 enhanced the barrier function by reducing permeability of the intestine through both transcellular and paracellular pathways. Cameron et al. (2005) also found that GLP-2 could reduce the bacterial penetration and colonic inflammatory cells in rats. The changes of the occludin, ZO-1 and claudin-1 mRNA expressions were determined to clarify the underlying mechanism of reducing the intestinal permeability and improving the barrier function by GLP-2 treatment. The results indicated that both 10 and 100 nmol/L additions of GLP-2 increased the claudin-1, occludin and ZO-1 mRNA expressions. Consistent with the gene observations, GLP-2 also stimulated the expressions of claudin-1, occludin and ZO-1. The results are consistant with Chen et al. (2008) who found that exogenous treatment of GLP-2 in obstructive jaundice rats increased the ZO-1, occludin, and claudin-1 expressions of intestinal mucosa. The results demonstrated that GLP-2 promoted the TJ of pigletsŌĆÖ intestine in the ablactating stress.
An additional in vitro tissue culture experiment was carried out to understand the molecular mechanisms of TJ expression changes associated with pigletsŌĆÖ ablactating. The results showed that both epithelial proteins and their mRNA expressions increased by adding GLP-2 to the culture medium. Besides, GLP-2 also increased phosphorylation of the mitogen-activated protein kinase ERK1/2 and its downstream target p90RSK. Similar results were also found in the neonatal pigletsŌĆÖ intestinal cells (Burrin et al., 2007) and rat kidney fibroblasts cells (Yusta et al., 1999). Activation of ERK1/2 has been proved to be important in the responses of GLP-2 increasing the intestinal cell proliferation in rats (Jasleen et al., 2000), humans (Margaret et al., 1998; Jasleen et al., 2002) and neonatal piglets (Burrin et al., 2007). However, the function of GLP-2 activating ERK1/2 has not been demonstrated before in weaned pigletsŌĆÖ tissue culture method in vitro.
GLP-2 could increase cell proliferation via the MAPK signaling pathway. Many research studies also revealed that the MAPK signaling pathway was able to modulate TJ paracellular transport due to the up or down regulatory effects on several TJ proteins expressions (Gonzalez et al., 2008). It is unknown whether GLP-2 also modulates the TJ protein expressions via the MAPK signaling pathway. U0126, a potent and specific mitogen-activated ERK-activating kinase inhibitor, was used to block the MAPK signaling pathway to explore whether GLP-2-induced ERK1/2 activation mechanistically links with an induction of TJ protein expressions. But once the ERK1/2 and p90RSK phosphorylation was decreased by U0126, the expressions of TJ proteins decreased significantly. In addition, the activation of ERK1/2 and subsequent p90RSK may involve the TJ protein expressions. A linkage between TJ proteins and barrier function in the intestine suggests that GLP-2 may modulate the intestinal barrier function via the ERK1/2 signaling pathway, although the effects of GLP-2 treatment on the permeability were not detected. However, other studies on various cells have shown that ERK1/2 activation is necessary for normal barrier function restoration. Wachtel et al. (2002) suggested that ERK1/2 activation was necessary for the induction of paracellular hyperpermeability under hypoxic conditions. In epithelia cells, it also has been observed that several growth factors such as transforming growth factor ╬▓ (Howe et al., 2005; Feldman et al., 2007), epithelial growth factor (EGF) (Basuroy et al., 2006) and HGF (Lipschutz et al., 2005) could activate the ERK1/2 to promote the barrier function of TJ.
In this experiment, the GLP-2 treatment significantly increased the ERK1/2, p90RSK, ZO-1, occludin, claudin-1 and their mRNA relative expressions and distribution. The TJ mRNA and protein expressions decreased significantly when U0126 was used to block the MAPK signaling pathway. These results suggested that GLP-2 improves intestinal tight junction expression in weaned pigs through MAPK signaling pathway.
ACKNOWLEDGEMENTS
This research was supported by Sichuan Provincial Education Department of Outstanding Academic and Technical Youth Leadership Fund (2010JQ0043). We are also grateful to Animal Biotechnology Center, Sichuan Agricultural University.
The work was supported by Sichuan Provincial Education Department of Outstanding Academic and Technical Youth Leadership Fund (2010JQ0043), China.
Figure┬Ā1
Effect of GLP-2 on tight junction proteins and signaling molecules mRNA expression. GLP-2, glucagon-like peptide 2. a,b,c Treatments with different letters are different at p<0.05.
Figure┬Ā2
Effect of GLP-2 on tight junction proteins mRNA expression with MAPK signaling pathway blocked by U0126. GLP-2, glucagon-like peptide 2; MARK, mitogen-activated protein kinase; ZO-1, zonula occludens-1. a,b,c Treatments with different letters are different at p<0.05.
Figure┬Ā3
Effect of GLP-2 on tight junction proteins and signaling molecules expression. They are shown as bar graphs. GLP-2, glucagon-like peptide 2; ZO-1, zonula occludens-1. a,b,c Treatments with different letters are different at p<0.05.

Figure┬Ā4
Effect of GLP-2 (100 nmol/L) on tight junction proteins and signaling molecules expression with MAPK signaling pathway blocked by U0126 (10 ╬╝mol/L). GLP-2, glucagon-like peptide 2; MARK, mitogen-activated protein kinase; ZO-1, zonula occludens-1. a,b,c Treatments with different letters are different at p<0.05.

Figure┬Ā5
Effect of GLP-2 (100 nmol/L) on distribution of tight junction proteins with MAPK signaling pathway blocked by U0126 (10 ╬╝mol/L). (400-fold increase) (OD). GLP-2, glucagon-like peptide 2; MARK, mitogen-activated protein kinase; ZO-1, zonula occludens-1. a,b,c Treatments with different letters are different at p<0.05.

Table┬Ā1
Defined porcine gene primers for PCR and qRT-PCR
REFERENCES
Basuroy S, Seth A, Elias B, Naren AP, Rao R. 2006. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J 393:69ŌĆō77.



Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. 2000. Glucagons-like peptide-2 enhances intestinal epithelial barrier function of both Tran cellular and Para cellular pathways in the mouse. Gut 47:112ŌĆō119.



Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. 2003. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 171:6164ŌĆō6172.


Burrin DG, Petersen Y, Stoll B, Sangild P. 2001. Glucagon-like peptide 2: A nutrient-responsive gut growth factor. J Nutr 131:709ŌĆō712.


Burrin DG, Stoll B, Guan XF, Cui LW, Chang XY, Hadsell D. 2007. GLP-2 rapidly activates divergent intracellular signaling pathways involved in intestinal cell survival and proliferation in neonatal piglets. Am J Physiol Endocrinol Metab 292:E281ŌĆōE291.


Cameron HL, Perdue MH. 2005. Stress impairs murine intestinal barrier function: improvement by glucagon-like peptide-2. J Pharmacol Exp Ther 314:214ŌĆō220.


Cameron HL, Yang PC, Perdue MH. 2003. Glucagon-like peptide-2-enhanced barrier function reduces pathophysiology in a model of food allergy. Am J Physiol Gastrointest Liver Physiol 284:G905ŌĆōG912.


Chen ZY, Feng XS, Yang P. 2008. Effect of glucagon-like peptide-2 on modulation of intestinal epithelium tight junction in rats with obstructive jaundice. Chi J Gener Surg 17:760ŌĆō763.
Drucker DJ, Yusta B, Boushey RP, DeForest BL, Brubaker PL. 1999. Human [Gly2] GLP-2 reduces the severity of colonic injury in a murine model of experimental colitis. Am J Physiol Gastrointest Liver Physiol 276:G79ŌĆōG91.

Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. 1998. The tight junction protein ZO-1 establishes a link between the Tran membrane protein occludin and the actin cytoskeleton. J Biol Chem 273:745ŌĆō753.
Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Richard AE, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. 1998. Identification of a novel inhibitor of mitogen activated protein kinas. J Biol Chem 273:18623ŌĆō18632.


Feldman G, Kiely B, Martin N, Ryan G, McMorrow T, Ryan MP. 2007. Role for TGF-beta in cyclosporine-induced modulation of renal epithelial barrier function. J Am Soc Nephrol 18:1662ŌĆō1671.


Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. 1993. Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol 123:1777ŌĆō1788.



Gonzalez-Mariscal L, Tapia R, Chamorro D. 2008. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys Acta 1778:729ŌĆō756.


Howe KL, Reardon C, Wang A, Nazli A, McKay DM. 2005. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am J Pathol 167:1587ŌĆō1597.



Jasleen J, Ashley SW, Shimoda N, Zinner MJ, Whang EE. 2002. Glucagon-like peptide 2 stimulates intestinal epithelial proliferation in vitro. Digest. Dis Sci 47:1135ŌĆō1140.

Jasleen J, Shimoda N, Shen ER, Tavakkolizadeh A, Whang EE, Jacobs DO, Zinner MJ, Ashley SW. 2000. Signaling mechanisms of glucagons-like peptide 2-induced intestinal epithelial cell proliferation. J Surg Res 90:13ŌĆō18.


Kansagra K, Stoll B, Rognerud C, Niinikoski H, Ching NO, Harvey R, Burrin D. 2003. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol 285:G1162ŌĆōG1170.


Kato Y, Yu D, Schwartz MZ. 1999. Glucagonlike peptide-2 enhances small intestinal absorptive function and mucosal mass in vivo. J Pediatr Surg 34:18ŌĆō21.


Kucharzik T, Walsh SV, Chen J. 2001. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol 159:2001ŌĆō2009.



Lipschutz JH, Li S, Arisco A, Balkovetz DF. 2005. Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. J Biol Chem 280:3780ŌĆō3788.


Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H, McCallum K, Sumner-Smith M, Drucker DJ, Prototypic AC. 1999. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc. Natl. Acad. Sci USA 96:1569ŌĆō1573.



Osek J. 1999. Prevalence of virulence factors of Escherichia coli strains isolated from diarrheic and healthy piglets after weaning. Vet Microbiol 68:209ŌĆō217.


Pfaffl MW. 2001. A new mathematical model for relative quantification in real time RT-PCR. Nucl. Acids Res. 29:9e45



Prasad R, Alavi K, Schwartz MZ. 2000. Glucagonlike peptide-2 analogue enhances intestinal mucosal mass after ischemia and reperfusion. J Pediatr Surg 35:357ŌĆō359.


Tsukita S, Furuse M, Itoh M. 2001. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285ŌĆō293.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





