 |
 |
| Anim Biosci > Volume 36(7); 2023 > Article |
|
Abstract
Objective
This study aimed to evaluate the physicochemical, metabolomic, and sensory qualities of Chikso and Hanwoo beef during 28 days of wet aging.
Methods
Rump and loins from Hanwoo and Chikso were obtained and wet-aged for 28 days at 4┬░C. The samples were collected at 7-day interval (n = 3 for each period). Physicochemical qualities including pH, meat color, shear force value, and myofibrillar fragmentation index, metabolomic profiles, and sensory attributes (volatile organic compounds and relative taste intensities) were measured.
Results
Chikso showed a significantly higher shear force value than Hanwoo on day 0; however, no differences between breeds were found after day 14, regardless of the cuts. Overall, Chikso had more abundant metabolites than Hanwoo, especially L-carnitine and tyrosine. Among the volatiles, the ketone ratio was higher in the Chikso rump than the Hanwoo rump; however, Chikso had fewer alcohols and aldehydes than Hanwoo. Chikso rump showed higher taste intensities than the Hanwoo rump on day 0, and sourness decreased in Chikso, but increased in the Hanwoo rump on day 14. Wet aging for 14 days intensified the taste of Chikso loin but reduced the umami intensity of Hanwoo loin.
Korean native cattle are classified as four indigenous cattle breeds: Hanwoo, Chikso, Heugu, and Jeju black [1]. Among them, Hanwoo is a well-known breed to consumers owing to its superior meat quality, such as excellent tenderness, high degree of marbling, and desirable flavor [2]. Hanwoo has low connective tissue and high intramuscular fat content, leading to high marbling quality, that is important for determining meat quality grade in Korea and other countries [3,4]. As a result, the great consumer demand for Hanwoo beef is attributed to its tenderness and flavor.
On the other hand, the beef quality of other cattle breeds, especially Chikso, has not well been evaluated. Despite the limited scientific information on Chikso beef quality, the rarity of Chikso beef in the market due to the limited population of approximately 4,000 heads in restricted areas makes it captivating to consumers [5]. Recently, Chikso has gained attention owing to its unique flavor characteristics to consumers who prioritize desirable meat flavor and seek new meat products [2]. Previous studies have reported flavor differences between cooked Hanwoo and Chikso beef through sensory assessment or instrumental analyses [4,5]. Wei et al [6] emphasized that the provision of beef products with flavor differences from a variety of cattle breeds is important to attract consumers with different preferences. Therefore, it is necessary to investigate Chikso beef quality and its differentiated characteristics from common Hanwoo beef to diversify Korean native cattle products in response to a variety of consumer demands.
According to the consumers and suppliers of Chikso beef, the toughness of Chikso beef has been pointed out as one of its drawbacks. As wet aging of beef has been widely adopted in the meat industry to improve eating quality, such as meat tenderization and flavor development [7,8], applying wet aging to Chikso beef could be an effective strategy for value addition through improving tenderness and flavor to enhance organoleptic attributes. Various flavor compounds and metabolite profiles in meat can be changed during wet aging [9,10]. As flavor is constituted of volatile organic compounds (VOC) and taste-active compounds, the quantitative analysis of these flavor compounds and metabolites that act as flavor precursors can help interpret beefŌĆÖs sensory quality [11ŌĆō13]. In addition, the development of beef flavor can be assessed by instrumental sensory analysis, such as electronic tongue and nose, which has the benefit of objective analysis [14,15]. Therefore, a 28-day wet aging process was applied to Chikso and Hanwoo rump, and loin cuts to investigate the effect of wet aging on meat quality. Additionally, the physicochemical quality, metabolomic profiles, and sensory attributes of Chikso rump and loin cuts during wet aging were evaluated and compared with those of Hanwoo beef.
Beef rump (M. semimembranosus) and loin (M. longissimus dorsi) were obtained from Hanwoo (32 months old, quality grade 1, 462 kg carcass weight) and Chikso (57 months old, quality grade 2, 373 kg carcass weight) at 48 h post-mortem, which were raised at the same condition (farm and diet). The age of each animal was determined based on its live weight in a commercial market. Visual fat and connective tissue were removed from the surface of the muscle, and each meat sample was cut into an average weight of 250 g.
To study the effect of wet aging on the Hanwoo and Chikso beef quality, rump and loin with an average weight of 250 g were vacuum-packaged (HFV-600L; Hankook Fujee Machinery Co., Ltd., Hwaseong, Korea) into low-density polyethylene/nylon bags (2 mL O2┬Ę(m2)ŌłÆ1┬Ę24 hŌłÆ1 at 0┬░C, 0.09 mm thickness; Sunkyung Co., Ltd., Seoul, Korea). The wet aging process was continued at 4┬░C for 28 days, and samples were collected at 7-day intervals (n = 3 for each wet aging period). At each wet aging period (days 0, 7, 14, 21, and 28), the beef samples were examined for color, and the samples were ground and used for physicochemical quality analyses. The remaining samples were vacuum packaged and stored at ŌłÆ70┬░C until further analyses.
The pH of the meat sample was measured according to Lee et al [16]. One gram of each sample was homogenized with 9 mL of deionized distilled water using a homogenizer (T25 digital ULTRA-TURRAX; Ika Works, Staufen, Germany) at 9,600 rpm for 30 s. Then, the homogenates were centrifuged at 2,265├Śg for 10 min (Continent 512R; Hanil Co., Ltd., Daejeon, Korea). The supernatants were filtered (No. 4; Whatman International Ltd., Kent, UK) and the pH of each sample was measured using a pH meter. A pH meter (Seven2Go; Mettler Toledo Inc., Schwerzenbach, Switzerland) was pre-calibrated by standardized buffer solutions (pH 4.01, 7.0, and 9.21) at room temperature.
Meat color was measured using a colorimeter (CM-5; Konica Minolta Censing Inc., Osaka, Japan) [16]. Before the analysis, the meat was bloomed for 30 min. Following pre-calibration of the colorimeter using a standard white plate, the analysis was conducted under the conditions of a D65 illuminant, a 10┬░ standard observer, and a plate with a diameter of 30 mm. The meatŌĆÖs lightness, redness, and yellowness were expressed as Commission Internationale dŌĆÖEclairage (CIE) L*, a*, and b* values, respectively. Six measurements were taken for each sample, and the average was used as one replicate.
The shear force value of Chikso and Hanwoo beef was measured according to Kim et al [17] with some modifications. Samples with an average weight of 100 g were placed in polyethylene bags in a water bath at 85┬░C until the internal temperature of the sample reached 72┬░C. After cooking, the samples were cooled to room temperature. Then, round cores were taken using a cork borer (1.27 cm diam.) parallel to the direction of the muscle fiber. A texture analyzer (TA1; AMETEK Lloyd Instruments Ltd., Fareham, UK) with a cross-head speed of 200 mm/min, a test speed of 60 mm/min, and a trigger load of 0.1 N was used.
The myofibrillar fragmentation index (MFI) analysis was conducted according to Kim et al [17] with a slight modification. The meat sample (1 g) was placed in the centrifugal tube and was homogenized at 15,000 rpm by adding 19 mL of MFI buffer (pH 7.0 at 4┬░C, containing 100 mM KCl, 1 mM ethylenediaminetetraacetic acid, 1 mM NaN3, and 25 mM potassium phosphate) for 30 s (T25 digital ULTRA-TURRAX; Ika Works, Germany). The homogenates were filtered through a 1-mm mesh strainer to remove connective tissues and washed with 10 mL of MFI buffer. The filtrate was centrifuged at 1,000├Śg for 15 min (Continent 512R; Hanil Co., Ltd., Korea). The supernatant was discarded, and the pellet was washed with 10 mL MFI buffer and vortexed. This experiment was repeated five times. Next, 10 mL of MFI buffer was added to the remaining pellet, vortexed, and the protein concentration was determined using the biuret method. The samples were then diluted with MFI buffer to reach 0.5 mg/mL of protein concentration. The absorbance of each sample was measured at 540 nm using a spectrophotometer (X-ma 3100; Human Co. Ltd., Seoul, Korea). The MFI value was calculated by multiplying the absorbance by 200.
The nuclear magnetic resonance (NMR) analysis was conducted according to Kim et al [18]. The meat samples (5 g) were homogenized with 20 mL of 0.6 M perchloric acid at 16,000 rpm for 1 min (T25 digital ULTRA-TURRAX; Ika Works, Germany). The homogenates were centrifuged at 2,265├Śg for 20 min (Continent 512R; Hanil Co., Ltd., Korea). The pH of the collected supernatants was adjusted to 7.0 at room temperature using perchloric acid and potassium hydroxide solutions. The samples were centrifuged under the same conditions. Afterward, the samples were filtered (No. 1; Whatman International Ltd., UK), and the filtrates were lyophilized (Freezer dryer 18; Labco Corp., Kansas City, MO, USA). Following lyophilization, 1 mL of 20 mM deuterium oxide-based phosphate buffer (pH 7.0) containing 1 mM 3-(trimethylsilyl) propionic-2, 2, 3, 3-d4 acid (TSP) was added to the lyophilized samples. The reconstituted samples were incubated at 37┬░C for 10 min and centrifuged at 2,265├Śg for 20 min (Continent 512R; Hanil Co., Ltd., Korea). The supernatants were further centrifuged at 17,000├Śg for 10 min (HM-150IV; Hanil Co., Ltd., Korea). The supernatants were loaded into NMR tubes and used for further analysis.
One-dimensional 1H NMR and 1H-13C heteronuclear single quantum coherence (HSQC) spectra of the samples were recorded using a Bruker 850 MHz cryo-NMR spectrometer (Bruker Biospin GmbH, Rheinstetten, Germany). The spectra were manually corrected by Chenomx NMR suite 7.1 (Chenomx, Inc., Edmonton, AB, Canada). The peak was identified by comparing the peak spectrum of the sample from HSQC using Topspin 4.0.8 (Bruker Biospin GmbH, Germany) with that of the Human Metabolome Database (www.hmdb.ca). The identified peaks were quantified through Chenomx NMR Suite 7.1. The identification and quantification of each metabolite were referenced to the resonance of the TSP.
VOC analysis was conducted using solid-phase microextraction and gas chromatography-tandem mass spectrometry (SPME-GC-MS/MS), according to Lee et al [16] with a slight modification. Ground meat samples (5 g) were placed into a 20-mL headspace vial and sealed with a PTFE-faced silicone septum. The samples were incubated at 40┬░C for 10 min before the extraction. A polydimethylsiloxane/divinylbenzene fiber (Supelco Inc., Bellefonte, PA, USA) was injected into the headspace of the vial and the extraction process was continued at 40┬░C for 30 min. The extracted VOCs were desorbed using a GC injector (Trace 1310; Thermo Fisher Scientific, Waltham, MA, USA) for 2 min at a split ratio of 1:10. A DB-Wax column (60 m├Ś0.25 mm i.d., and 0.50 ╬╝m film thickness; Agilent Technologies Inc., Santa Clara, CA, USA) was equipped to the GC for VOCs separation. The GC condition was as follows: helium was used as the carrier gas at the flow rate of 1.5 mL/min; initial oven temperature was held at 40┬░C for 2 min, increased at a rate of 4┬░C/min and held at 150┬░C for 10 min, then increased at a rate of 4┬░C/min and held at 200┬░C for 5 min, and finally, increased at a rate of 10┬░C/min and held at 230┬░C for 5 min. Mass spectra of the fragmentations were obtained using a triple quadrupole mass spectrometer (TSQ 8000; Thermo Fisher Scientific, USA) in the electron impact (EI) mode with a scan range from 35 to 550 m/z in full-scan mode at 0.2 s of scan interval. VOCs in Chikso and Hanwoo beef were identified by comparing their mass spectra with those from the National Institute of Standards and Technology (NIST) mass spectral library (version 2.0 g) and the linear retention index (LRI). For the LRI, a n-alkane standard (C8ŌĆōC20) was analyzed under the same conditions for the calculation of the LRI of each VOC.
Electronic tongue analysis was performed according to Lee et al [14] with slight modifications. The ground meat samples (5 g) were homogenized with the addition of 100 mL deionized distilled water at 10,000 rpm for 30 s (T25 digital ULTRA-TURRAX; Ika Works, Germany). The samples were centrifuged at 2,265├Śg for 10 min (Continent 512R; Hanil Co., Ltd., Korea) and the supernatants were filtered (No. 4; Whatman International Ltd., UK). Taste attributes of the samples were analyzed using an electronic tongue (Astree; Alpha MOS, Toulous, France). Taste screening was performed using Alpha soft (Alpha MOS, France) to compare the relative taste intensities of the samples using the following equation:
where X and m are the mean sensor values for each and the total group, respectively, and Žā represents the deviation of the sensor value within the total group. Relative taste intensity was measured on a 10-point scale. For the AHS and NMS sensors, the value
X - m Žā
All experiments were conducted in triplicate, and statistical analyses were performed via one-way analysis of variance using a linear model (SAS 9.4, SAS Institute Inc., Cary, NC, USA). Significant differences between treatments were determined using TukeyŌĆÖs multiple comparison test (p<0.05). The results are expressed as the mean values and standard error of the mean. Correlation analysis was performed with SAS 9.4 (SAS Institute, Inc., USA). The heat map was visualized using TBtools v0.6735 (www.github.com/CJ-Chen/TBtools). For multivariate analysis, orthogonal partial least squares-discriminant analysis (OPLS-DA) and partial least squares-discriminant analysis (PLS-DA) were performed using MetaboAnalyst 5.0 (www.metaboanalyst.ca). OPLS-DA was performed between Hanwoo and Chikso beef for the entire wet aging period (days 0 to 28) to identify universally differentiated metabolites and VOC profiles between the two breeds. PLS-DA between Hanwoo and Chikso beef at each wet aging period was performed to study the effects of breeds and wet aging on metabolites and VOC changes.
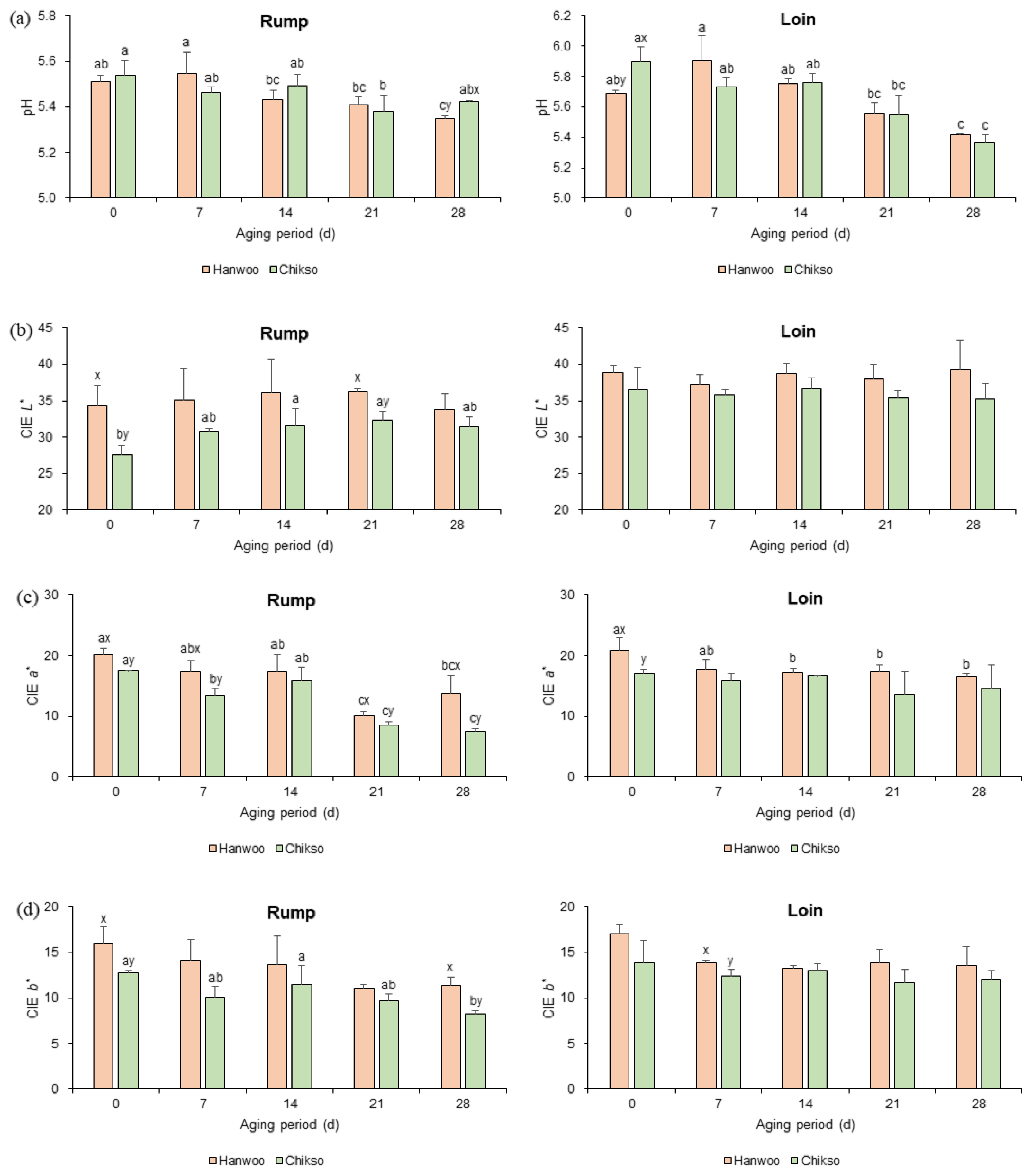
The pH of Hanwoo and Chikso rumps on day 0 was not significantly different, and they gradually decreased until day 21 (Figure 1a). However, on day 28, the Chikso rump showed a significantly higher pH than the Hanwoo rump. Generally, the meat pH drops during wet aging because of the accumulation of lactic acid by the increase in lactic acid bacteria under anaerobic conditions [11]. On the contrary, the generation of alkaline products such as ammonia and amines by protein hydrolysis during wet aging would increase the pH [19]. Meanwhile, Chikso loin had a significantly higher pH compared to Hanwoo loin on day 0. However, no significant difference in loin pH was observed between the two breeds after wet aging.
During wet aging, the L* value of the Chikso rump significantly increased on days 14 and 21 compared to day 0 (Figure 1b). The increase in L* value in wet-aged beef may be attributed to the expulsion of moisture at the meat surface, which reflects the light, modification of surface protein structure by hydrolysis, denaturation during wet aging, etc. [7,20]. On the other hand, the Hanwoo rump had not exhibited any difference in L* value during 28 days of wet aging.
During wet aging, a* values of rump from both breeds significantly decreased (Figure 1c), following the results of the study performed by Ma et al [21]. This was possibly due to the reduced color stability by the oxidation of myoglobin and lipids [20]. It was also reported that extended vacuum storage would deteriorate color stability and bloom development of meat [21]. Meanwhile, a major difference in meat color between Hanwoo and Chikso rump was found in a* value. The Hanwoo rump showed a redder surface than the Chikso rump during wet aging (p<0.05), except on day 14. The a* value is highly affected by the content and chemical status of myoglobin [22]. Furthermore, recent studies found a negative correlation between color stability and phenylalanine or tryptophan, which act as substrates for reactive oxygen species, suggesting that metabolomic changes might influence the meat color [12,22]. In the present study, Chikso rump had higher phenylalanine content during wet aging compared to Hanwoo rump (Table 1), and phenylalanine was negatively correlated with a* value (r = ŌłÆ0.80; Supplementary Table S3). Therefore, it can be assumed that the high phenylalanine content in Chikso rump could decrease the a* value during storage.
The b* value in the Chikso rump decreased during wet aging (p<0.05), whereas there was no significant difference in Hanwoo rump (Figure 1d). However, the Hanwoo rump had a higher b* value on days 0 and 28 than the Chikso rump. In contrast, there were fewer changes in the color of Hanwoo and Chikso loins during wet aging.
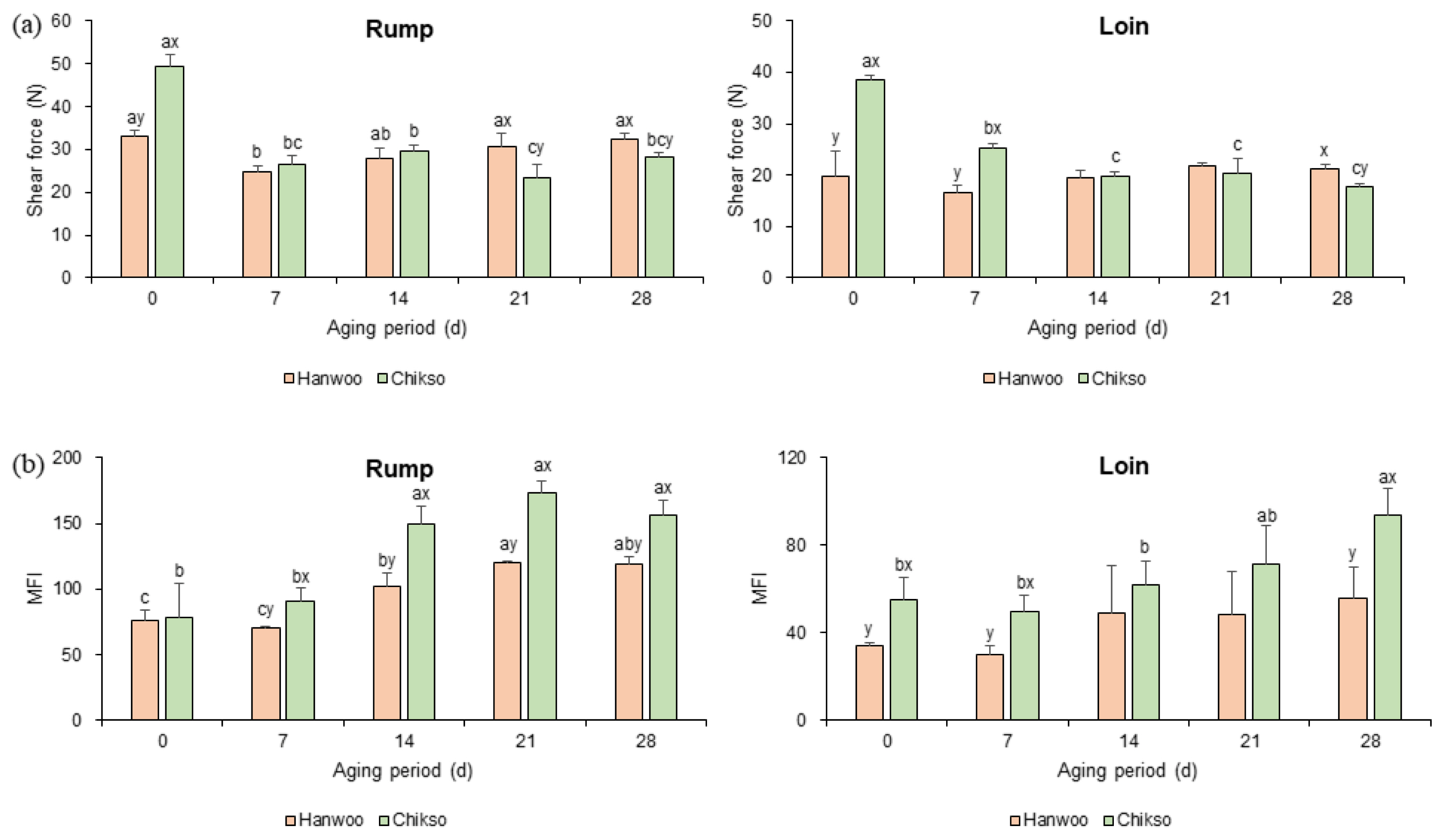
Before wet aging, Chikso beef showed a significantly higher shear force value than Hanwoo rump (Figure 2a). Similarly, the shear force value of Chikso loin was higher than that of Hanwoo loin on day 0 (p<0.05). Hanwoo cattle have been bred for a long time to enhance meat tenderness, intramuscular fat content, marbling score, etc. [23]. As a result, Hanwoo beef has been recognized as tender beef with high meat quality grade, thin muscle fibers, and connective tissues [5]. However, the muscle characteristics of Chikso beef have not been well studied. It has been reported that the differentially differentiated genes between Hanwoo and Chikso were associated with adipose tissue development, fatty acid metabolism, and muscle lipid composition [24]. Therefore, differences in the characteristics of muscle tissue of Hanwoo and Chikso beef might be a possible candidate for explaining their different tenderness.
Nonetheless, as wet aging proceeded, the shear force value of the Chikso rump decreased to almost half of the original value on day 21. No significant difference was found between the Hanwoo and Chikso rumps after day 7. The Chikso rump showed lower shear force values on days 21 and 28 than the Hanwoo rump (p<0.05). The decrease in shear force value during wet aging may be attributed to the action of proteolytic enzymes [10]. Moreover, the effect of wet aging on meat tenderization was more remarkable in Chikso rump than in Hanwoo rump. Based on the standard of Belew et al [25] Chikso and Hanwoo rump on day 0 could be classified as ŌĆ£toughŌĆØ (>45 N) and ŌĆ£tenderŌĆØ (between 31 and 38 N), respectively. However, after wet aging process, the shear force values of rump cut from two breeds showed similar values with the same category of ŌĆ£very tenderŌĆØ (<31 N), suggesting that Chikso rump might have similar tenderness properties with Hanwoo rump after day 7 of wet aging.
Similarly, the shear force values of the Chikso loin was significantly higher than the Hawnoo loin in the early phase of wet aging (days 0 to 7). However, as the wet aging process extended to day 14, the shear force value of the Chikso loin reached a value similar to that of the Hanwoo loin (p>0.05). Then, it significantly decreased and became lower than that of the Hanwoo loin on day 28. However, unlike the Chikso loin, there was no significant change in the shear force value of the Hanwoo loin during the wet aging period. Initially, Chikso loin could be categorized as ŌĆ£intermediateŌĆØ (between 38 and 45 N) on day 0 [25]. Nonetheless, its shear force value reduced below 31 N after day 7 and showed insignificant values with Hanwoo loin on day 14, indicating that 14 days of wet aging might lead Chikso loin to show similar tenderness with Hanwoo loin.
Meat tenderness is mainly determined by the connective tissue content, myofibrillar protein degradation after slaughter, and sarcomere length [23,26]. Among them, the degree of myofibril degradation can be assessed by MFI. Especially, MFI value is more direct than shear force value for estimating the tenderization effect of wet aging on meat product [27]. In the present study, the MFI values of Hanwoo and Chikso rumps significantly increased with the wet aging period (Figure 2b), indicating that wet aging had a positive effect on the tenderization of Hanwoo and Chikso beef. Especially, the MFI value of Chikso rump was significantly higher than that of the Hanwoo rump after day 7. In the case of loin cuts, the MFI value of Chikso loin significantly increased, whereas that of Hanwoo loin did not change during wet aging. The results showed that Chikso beef with low tenderness on day 0 was significantly improved by wet aging. Furthermore, aging was more effective for Chikso beef than for Hanwoo beef. Therefore, wet aging for 14 days or more may contribute to the value addition of Chikso beef by tenderizing it to make the tenderness similar to that of Hanwoo beef.
A total of 27 metabolites were identified in rump and loin from both breeds during 28 days of wet aging, including 17 free amino acids, dipeptides and their derivatives, four nucleotides and nucleosides, four organic acids, and others (ethanol and niacinamide; Tables 1ŌĆō4).
On day 0, Chikso rump had a higher abundance of tyrosine, L-carnitine, o-acetylcarnitine, fumarate, and ethanol than Hanwoo rump (Tables 1 and 2; p<0.05). As wet aging proceeded, the amounts of free amino acids, dipeptides, and derivatives increased significantly in both Hanwoo and Chikso rumps owing to the myofibrillar protein degradation (Table 1). In particular, higher amounts of alanine, asparagine, glutamate, glycine, isoleucine, leucine, methionine, phenylalanine, tyrosine, valine, and L-carnitine were found in the Chikso rump than in the Hanwoo rump (p<0.05). A higher MFI in the Chikso rump during wet aging might lead to higher amounts of free amino acids, dipeptides, and derivatives compared to the Hanwoo rump (Figure 2b). Among them, free amino acids can be utilized as flavor precursors for both VOC production and taste-active compounds [14,28]. Hence, metabolomic differences lead to the flavor differentiation between two breeds.
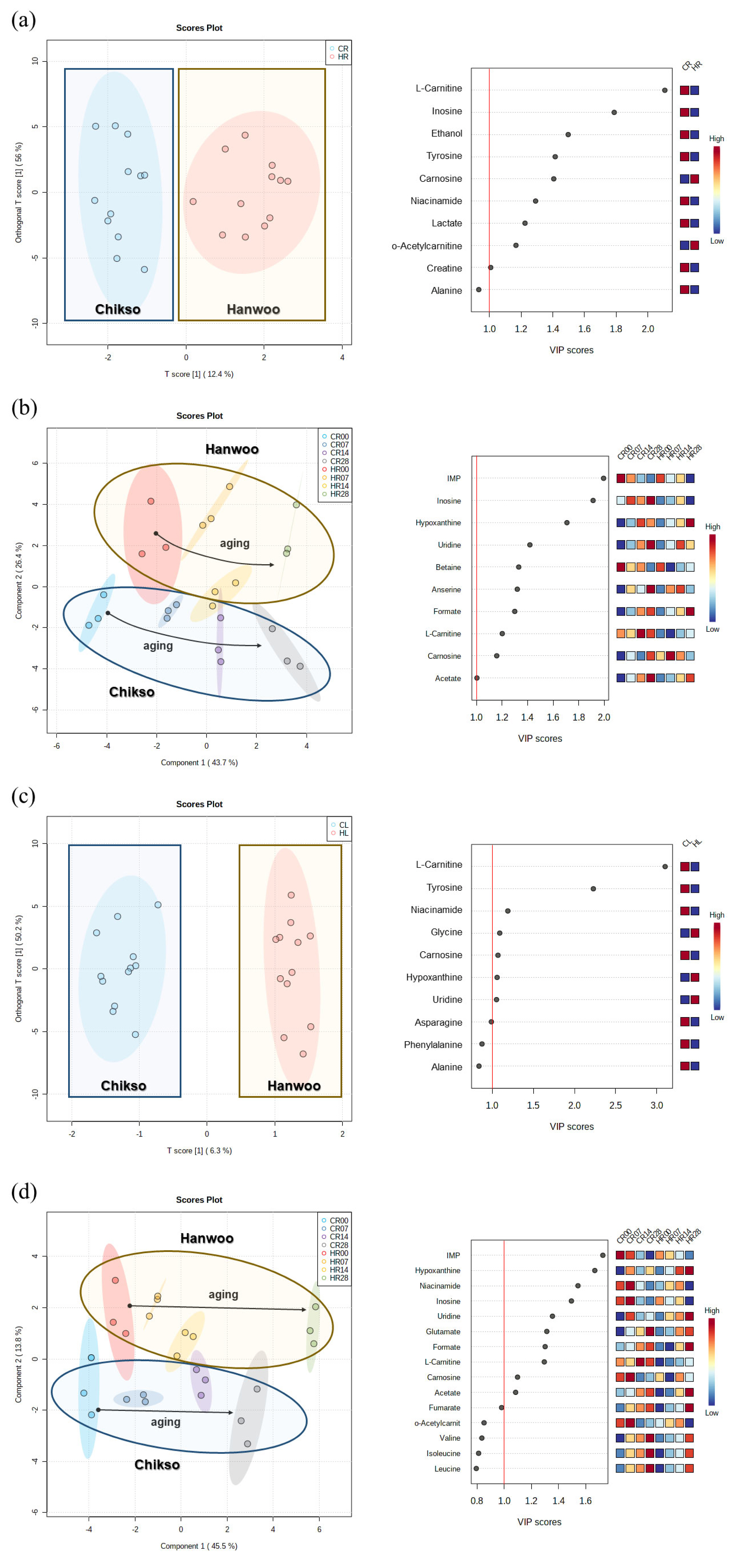
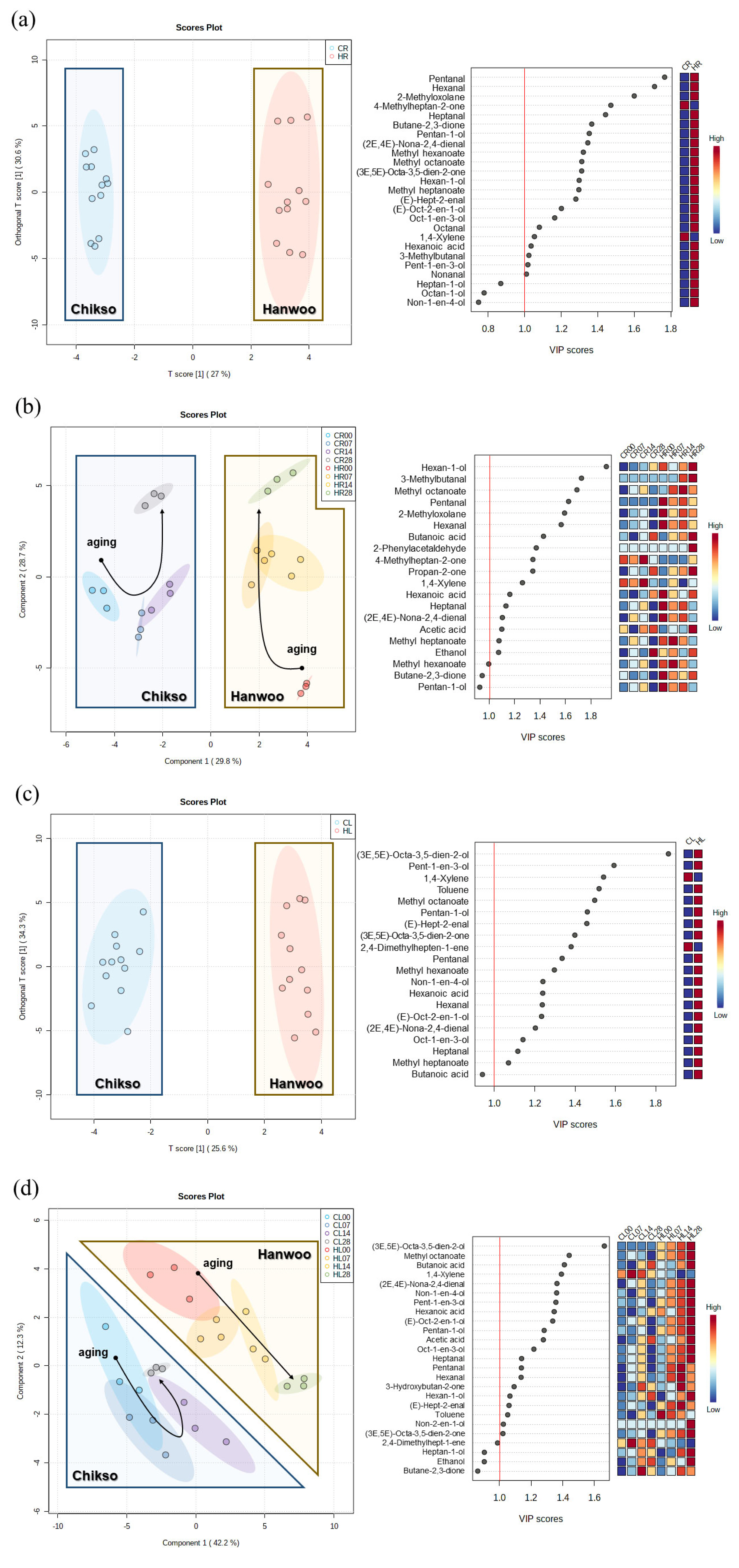
The metabolomic profiles of the Hanwoo and Chikso rumps measured during the entire wet aging period were separated in the OPLS-DA plot, and major metabolomic differences between the two breeds were found to be L-carnitine, inosine, ethanol, tyrosine, carnosine, niacinamide, lactate, o-acetylcarnitine, and creatine, which had variable importance in projection (VIP) score higher than 1 (Figure 3a). L-carnitine is a bioactive compound that is responsible for fatty acid metabolism [29]. o-Acetylcarnitine in beef rump showed negative correlations with sour and bitter taste intensities measured via electronic tongue (Supplementary Table S4). Tyrosine is associated with a bitter taste; however, it could also enhance the umami intensity when salts and free acidic amino acids are present [8,30]. Meanwhile, the effects of both breed and wet aging period on the change in metabolomic profiles in beef rump were also explored through PLS-DA (Figure 3b). In the PLS-DA plot, both Hanwoo and Chikso rumps moved in a positive direction on the X axis during wet aging. The separation of the two breeds was attributed to the presence of free amino acids, L-carnitine, o-acetylcarnitine, and inosine. inosine 5ŌĆ▓-monophosphate (IMP) and inosine, which had VIP>1 and thus had a great influence on the differentiation of sample groups in the PLS-DA plot, were also more abundant in the Chikso rump than in the Hanwoo rump (p<0.05). IMP can also contribute to the umami flavor of meat in addition to glutamate [13]. Therefore, higher amounts of glutamate and IMP in Chikso rump may act as umami flavor enhancers. In particular, the IMP content was negatively correlated with the intensity of the NMS sensor in the electronic tongue, suggesting the action of IMP in improving the umami taste of beef rump (r = ŌłÆ0.54; Supplementary Table S4). Overall, various metabolites were significantly increased in the Chikso rump during the wet aging process compared to the Hanwoo rump, which indicates that the sensory attributes of the Chikso rump may be enhanced by the increase in flavor precursors such as L-carnitine, o-acetylcarnitine, tyrosine, IMP, and inosine, and therefore be more distinguishable from the Hanwoo rump after wet aging.
In the loin cut, only the fumarate and ethanol contents were significantly higher in Chikso than in Hanwoo on day 0 (Table 4). However, on day 7, most metabolites, such as alanine, asparagine, isoleucine, leucine, phenylalanine, tyrosine, valine, anserine, carnosine, creatine, L-carnitine, o-acetylcarnitine, hypoxanthine, IMP, and inosine, were higher in Chikso loin than in Hanwoo loin (Tables 3 and 4; p<0.05). Remarkably, tyrosine and L-carnitine contents were significantly higher in the Chikso loin than in the Hanwoo loin during most of the wet aging period.
In the OPLS-DA plot, the major components that contributed to the separation between the two breeds included L-carnitine, tyrosine, niacinamide, glycine, carnosine, hypoxanthine, and uridine, which had a VIP>1 (Figure 3c). In particular, L-carnitine and tyrosine showed relatively high values of VIP scores, not only in the Chikso loin but also in the Chikso rump, indicating that these metabolites might be representative compounds in the Chikso beef. In the PLS-DA plot, metabolites in both Hanwoo and Chikso loins generally increased during the wet aging period (Figure 3d). As a result, both Hanwoo and Chikso loin moved in a positive direction along the X axis during the wet aging period in the PLS-DA plot. Similar to the results for beef rump, nucleosides and nucleotides, such as IMP, hypoxanthine, and inosine, were the major contributors to the separation of groups (VIP>1) in the PLS-DA plot. From the results, the differences in the metabolomic profiles between Hanwoo and Chikso beef, such as L-carnitine and tyrosine were observed. These differences may lead to the differentiation of sensory attributes between Hanwoo and Chikso beef.
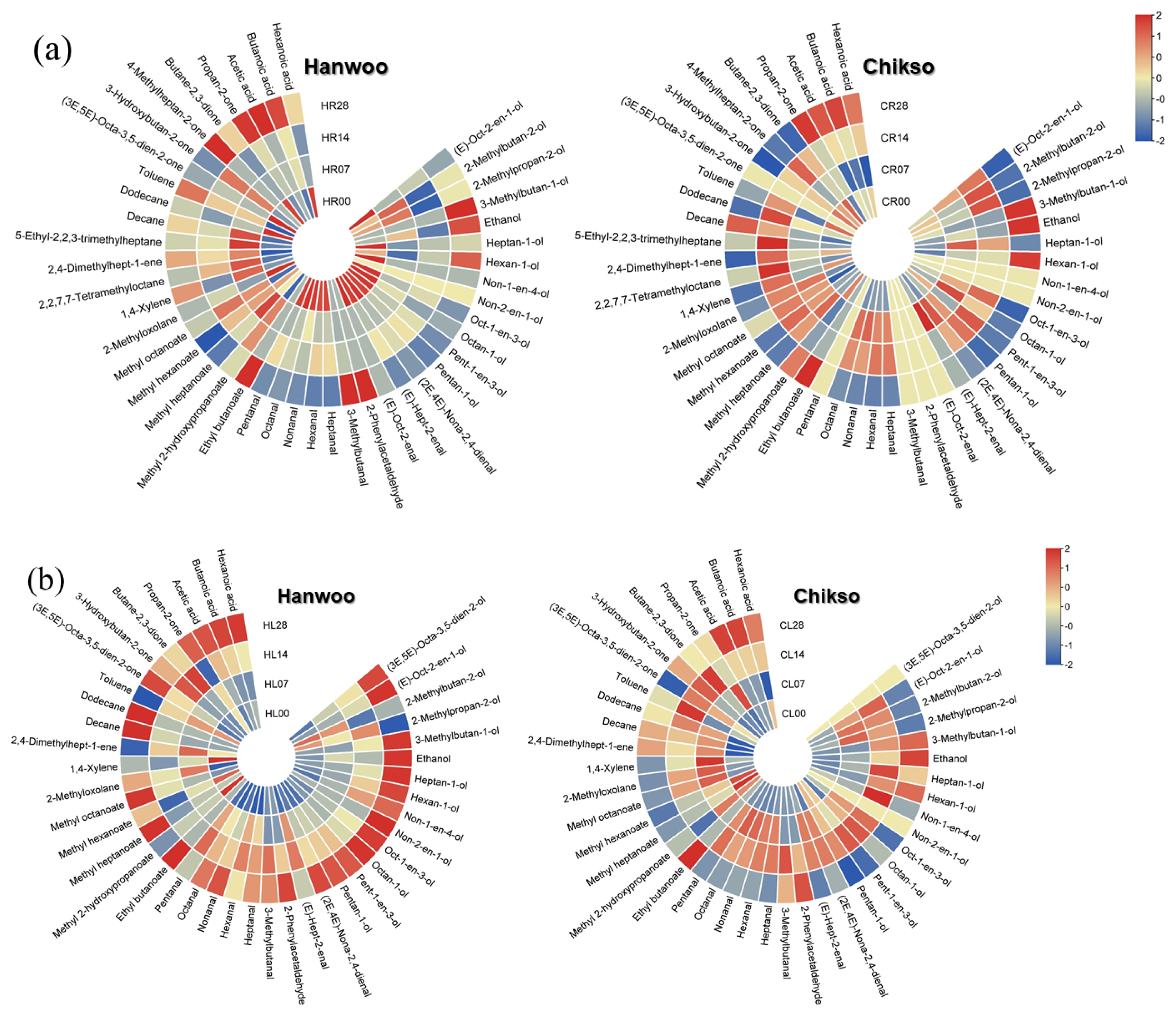
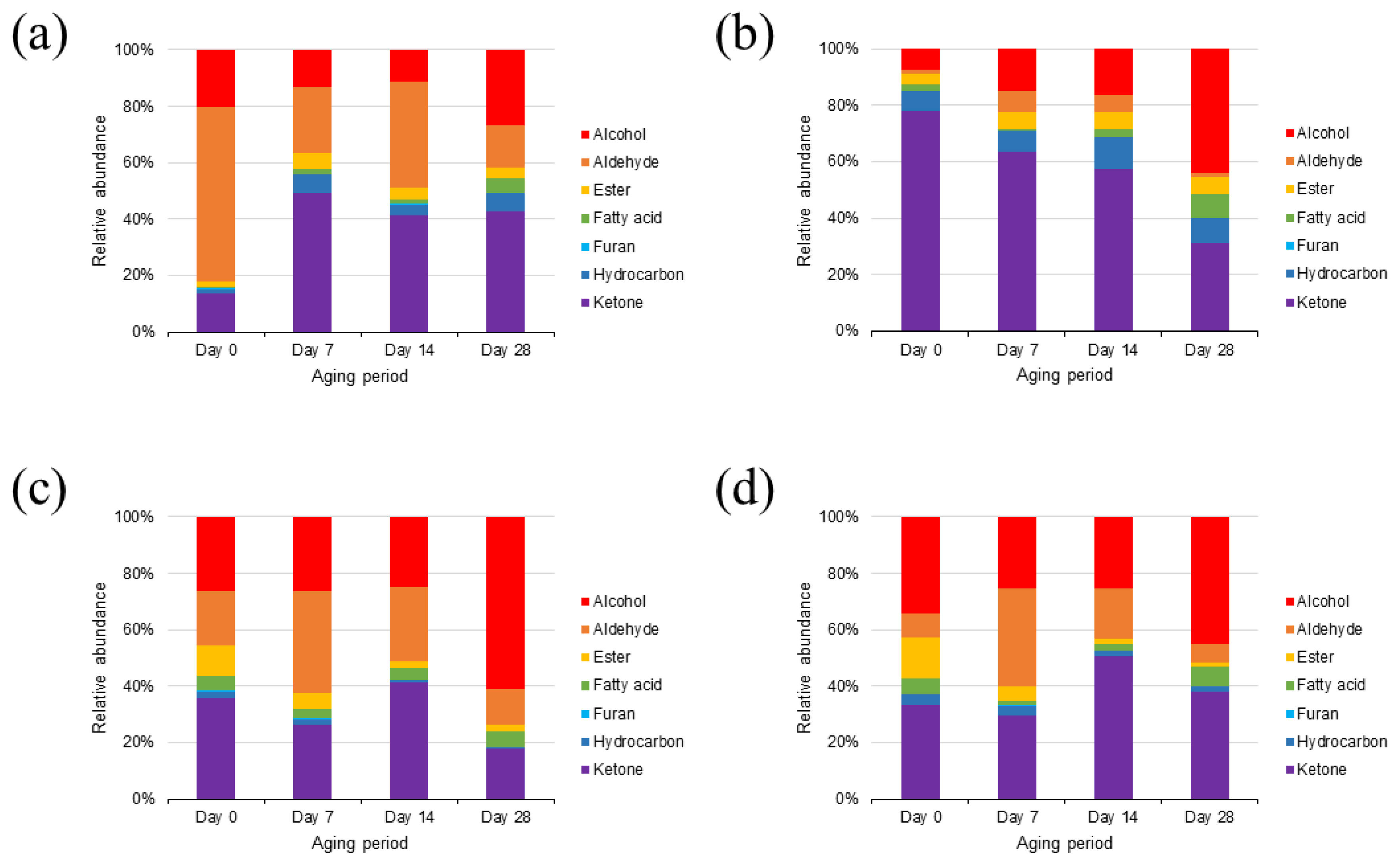
A total of 43 VOCs were identified in the rump cuts during wet aging, including 12 alcohols, 10 aldehydes, 5 esters, 1 furan, 7 hydrocarbons, 5 ketones, and 3 volatile fatty acids (Figure 4). Most of the VOCs detected in this study have been reported as compounds produced by lipid oxidation [3]. Interestingly, on day 0, the major VOC group in the Hanwoo rump was aldehydes, whereas that in the Chikso rump was ketones (Figure 5a, b). In general, aldehydes are known as the major contributors to beef flavor among lipid oxidation-derived VOCs [7]. However, ketones also exhibit distinct oily, fruity, and fatty flavors with low odor-thresholds [11,31]. Therefore, these compounds could contribute significantly to the volatile flavor of Chikso rump. Ketones are known to be generated by amino acid degradation, lipid oxidation, carbohydrate metabolism or ╬▓-keto acid oxidation [32]. In particular, the amount of 3-hydroxybutan-2-one was significantly higher in the Chikso rump than in the Hanwoo rump, and it was the most abundant compound in the Chikso rump on day 0 (Supplementary Table S1). Therefore, 3-hydroxybutan-2-one is considered a potential contributor to the unique flavor of Chikso rump. Furthermore, 4-methylheptan-2-one was present only in the Chikso rump until day 21. In contrast, other VOC groups, such as alcohols, aldehydes, and esters, were present at higher concentrations in the Hanwoo rump than in the Chikso rump. Hexanal was the most abundant compound in the Hanwoo rump. It is a product of linoleic acid oxidation and is a major VOC in beef that gives a pleasant flavor to cooked meat [7,8]. Moreover, the degradation products of oleic acid, such as heptanal, octanal, and nonanal, were also detected in the Hanwoo and Chikso rumps. These compounds have sweet and fatty odor characteristics and influences the beef flavor significantly [11,33]. Differences in the lipid oxidation-derived VOCs contents, such as hexanal, heptanal, octanal, and nonanal, between Hanwoo and Chikso rump might be due to differences in antioxidant activity. We found that Chikso rump had significantly higher 2,2ŌĆ▓-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity than Hanwoo rump on days 7, 21, and 28 (Supplementary Figure S1). This might help reduce the extent of lipid oxidation, as major lipid oxidation-derived products (hexanal and heptanal) were less abundant in the Chikso rump than in the Hanwoo rump.
As the wet aging period increased, the alcohol percentage also increased, while that of ketones decreased in the Chikso rump. Ethanol can be generated by microbial fermentation during the aging process [11]. Other compounds, (E)-oct-2-en-1-ol and hexan-1-ol, in the Chikso rump also amplified during aging (p<0.05). However, these compounds have high odor thresholds, and thus contribute less to beef flavor. There was also a significant rise in the aldehyde and ester contents in the Chikso rump until day 14 and then reduced on day 28. On the other hand, volatile fatty acids in the Chikso rump significantly decreased on day 7 but increased thereafter. Wet aging enhances meat flavor by generating flavor precursors and VOCs; however, previous studies have also reported a decrease in major volatile flavor contributors, such as aldehydes, during wet aging. Xu et al [8] reported that the octanal and nonanal concentrations decreased as the wet aging period increased. Similarly, Lee et al [11] reported that the concentration of esters in beef decreased after day 28. In the present study, the total amount of VOCs in both Hanwoo and Chikso rumps decreased after day 28 (Supplementary Table S1). The overall VOC profiles of Hanwoo and Chikso rump were compared using OPLS-DA to identify universal differences between the two breeds (Figure 6a). Two breeds were clearly differentiated which meant that the VOC profiles of Hanwoo and Chikso rump were different. Wei et al [6] reported that the difference in the VOC of different breeds of cattle might be attributed to the fatty acid composition or intramuscular fat content, derived from the genetic difference in lipid metabolism. Furthermore, numerous metabolites including amino acids and nucleotides participate in flavor formation, and therefore the difference in metabolites may also affect the change in VOC profiles of beef [30]. While most VOCs with a VIP>1 were more abundant in the Hanwoo rump than in the Chikso rump, the contents of 4-methylheptan-2-one and 1, 4-xylene were higher in the Chikso rump. During wet aging, the Hanwoo rump showed more remarkable VOC changes than the Chikso rump (Figure 6b).
A total of 41 VOCs were identified in the Hanwoo and Chikso loins (Supplementary Table S2). It is composed of 14 alcohols, 9 aldehydes, 5 esters, 1 furan, 5 hydrocarbons, 4 ketones, and 3 volatile fatty acids. Most VOCs were fewer in the Chikso loin than in the Hanwoo loin. Instead, 1, 4-xylene and 2, 4-dimethylhepten-1-ene were generally more abundant in the Chikso loin throughout the wet aging period than in the Hanwoo loin (Figure 6c).
During wet aging, Hanwoo loin showed a general increase in VOCs; however, Chikso loin only showed a gradual rise in alcohols, but the amounts of aldehydes and esters decreased after 28 days of wet aging (Figure 5), indicating that these two breeds showed different trends in VOC profile changes during wet aging (Figure 6d). Considering the origin of these VOCs, the degree of lipid oxidation might influence the volatile flavor characteristics of Hanwoo and Chikso loins. Chikso loin showed higher antioxidant activities (ABTS radical scavenging activity and ferric reducing antioxidant power) than Hanwoo loin on day 0, which might explain the overall lower quantities of lipid oxidation-derived VOCs in Chikso loin than in Hanwoo loin (Supplementary Figure S1). Overall, VOCs decreased gradually in the Chikso rump during wet aging, while 14 days of wet aging led to abundant VOC content in the Chikso loin.
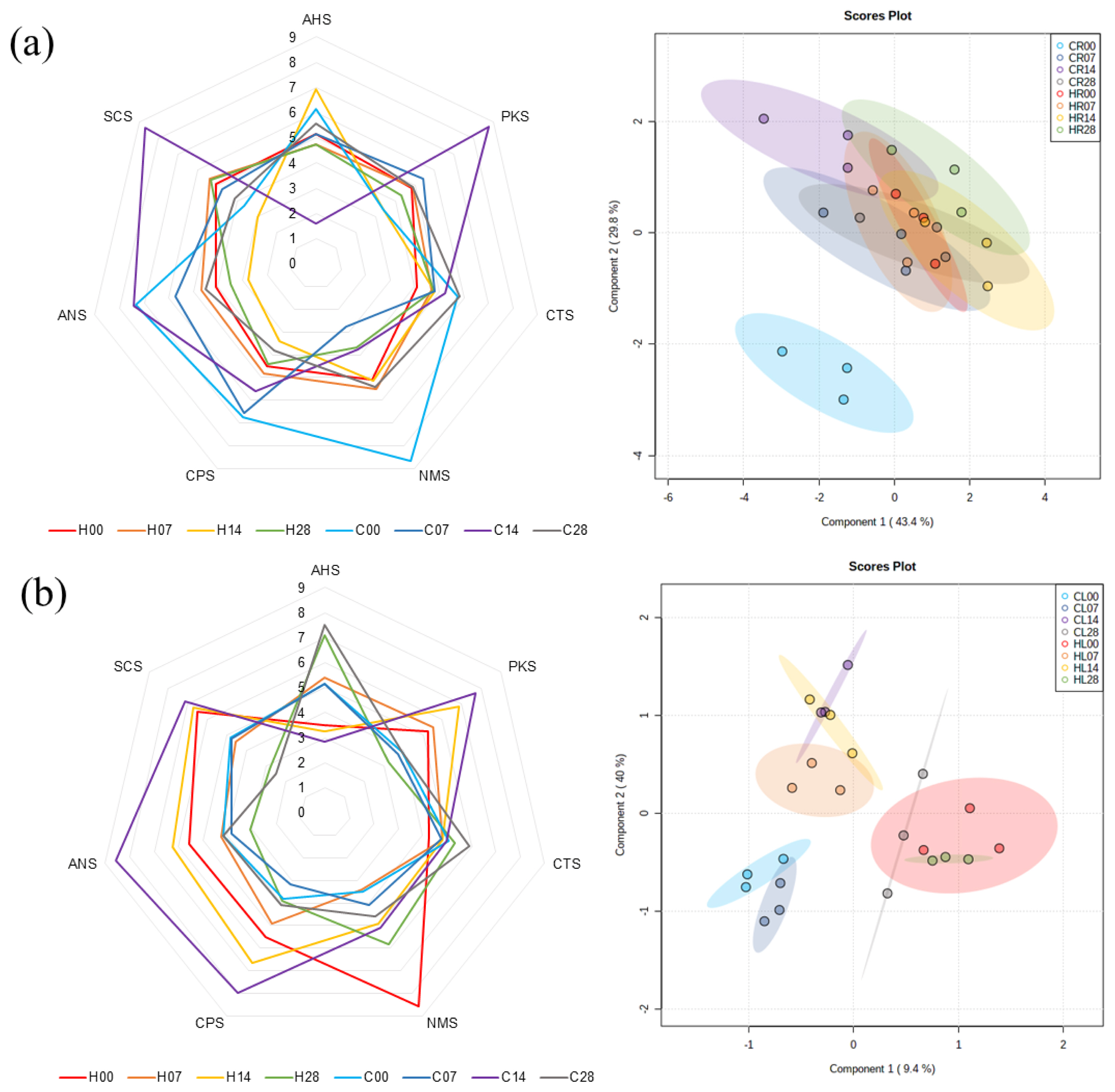
Electronic tongue analysis was conducted using seven sensors for Hanwoo and Chikso beef on days 0, 7, 14, and 28 (Supplementary Figure S2). The sensors AHS, CTS, NMS, ANS, and SCS respond to sour, salty, umami, sweet, and bitterness, respectively, whereas PKS and CPS represent universal taste intensity. The PLS-DA plot for the electronic tongue results showed that the taste characteristics of the Chikso rump on day 0 were clearly separated from those of Hanwoo rumps on days 0 to 28 and Chikso rumps on days 7 to 28 (Figure 7a). The characteristics of the Chikso rump on day 0 were high values of relative taste intensity for NMS and CPS, indicating a higher intensity for umami taste and universal taste, respectively. In addition, Chiko rump on day 0 showed overall high relative taste intensities, except SCS (bitterness) and PKS (universal), compared to Hanwoo rump on day 0. Umami taste is mainly derived from umami amino acids (aspartic and glutamic acid) and nucleotides (IMP and guanosine 5ŌĆ▓-monophosphate [GMP]) [34]. Particularly, the synergistic effect of IMP and glutamic acid could improve the strength of umami taste considerably [8]. Here, the IMP content was the highest in Chikso rump on day 0 of wet aging (Table 2), and a negative correlation between IMP and sensor intensity of NMS was identified (r = ŌłÆ0.54; Supplementary Table S4). The lower sensor intensity of NMS indicates a stronger taste intensity for umami taste, and therefore, high content of IMP in Chikso rump on day 0 might contribute to the high umami intensity in electronic tongue analysis.
Additionally, the Chikso rump on day 14 also had different characteristics than the other groups; it showed high values of taste intensity for ANS, SCS, and PKS, which correspond to sweetness, bitterness, and universal taste, respectively. Lee et al [14] reported that dry and wet aging of beef led to the increase of umami taste intensity. However, in our study, umami intensity of Chikso rump decreased considerably from day 0 to 7, although it gradually increased afterwards. Previous studies have reported that the taste characteristics of alanine, glutamine, and glycine impart sweetness, while isoleucine, leucine, phenylalanine, tyrosine, and valine could contribute to bitterness [9]. Moreover, reducing sugars can contribute to the sweetness of the meat, and anserine, carnosine, and nucleosides such as inosine and hypoxanthine provide bitterness [14,30]. In this study, the contents of most of the above-mentioned metabolites were significantly increased during wet aging, which would lead to high intensity for sweetness and bitterness. Especially, considering that umami intensity decreased while bitterness increased dramatically during 14 days of wet aging in Chikso rump, the degradation of umami contributors such as IMP which generated inosine and hypoxanthine at the early phase of wet aging might influence the change of taste attributes of Chikso rump considerably during wet aging. Previous study also noted that PKS is used for the general purpose of taste differentiation in electronic tongues [8], and the relatively high intensity of the Chikso rump on day 14 for the PKS sensor indicates that the universal taste of the Chikso rump on day 14 may be distinguishable from others. In contrast, wet aging for 14 days in Hanwoo rump increased sour taste intensities but lowered other taste intensities. The increase of sourness in wet-aged beef might derive from the accumulation of lactate by the action of lactic acid bacteria [35]. During wet aging for 14 days, lactate content was significantly increased in Hanwoo rump (Table 2).
The PLS-DA plot for taste characteristics of Hanwoo and Chikso loins revealed that loins from the two breeds showed a clear separation on day 0 (Figure 7b). In particular, higher umami intensity was found in Hanwoo loin on day 0 than in Chikso loin. Multivariate analysis also implied that the wet aging period of 14 days for Chikso loin would lead to different taste attributes compared with days 0, 7, or 28. Chikso loin on day 14 showed higher values of ANS, SCS, PKS, and CPS sensor intensities among treatments, indicating a strong intensity of sweetness, bitterness, and universal taste. In contrast, Chikso and Hanwoo loin on day 28 led to high sour attributes, possibly owing to the increased amount of organic acids including acetate, formate or fumarate (p<0.05). This was possibly due to increased alanine, phenylalanine, tyrosine, acetic acid, and formic acid in meat wet-aged for a long period [8,30]. The results suggested that the umami taste intensity was high in Chikso rump and Hanwoo loin on day 0. In contrast, the wet aging process for 14 days was effective in enhancing overall taste intensities and taste-active compounds in the Chikso beef.
Overall, major differences between Hanwoo and Chikso beef on day 0 were observed in the CIE a* value, shear force value, VOCs, and taste attributes. In particular, the higher shear force value in Chikso beef than in Hanwoo beef indicates the need for the wet aging process for meat tenderization. Wet aging decreased the shear force value and increased the MFI of Chikso rump and loin considerably, and it also increased flavor compounds, such as free amino acids and alcoholic VOCs. Furthermore, 14 days of wet aging enhanced the taste intensity of Chikso beef. Therefore, the quality of Chikso beef changed during wet aging, and the wet aging period of 14 days was recommended to improve tenderness, similar to Hanwoo beef, while enhancing its flavor characteristics.
Notes
SUPPLEMENTARY MATERIAL
Supplementary file is available from: https://doi.org/10.5713/ab.23.0001
Supplementary Figure S1. Antioxidant activities of Hanwoo and Chikso beef cuts during aging period.
ab-23-0001-Supplementary-Fig-1.pdf
Supplementary Figure S2. Sensor value of seven electronic tongue sensors for Hanwoo and Chikso rump (a) and loin (b) during aging, respectively.
ab-23-0001-Supplementary-Fig-2.pdf
Supplementary Table S1. Volatile flavor compounds (area unit ├Ś106) in Hanwoo and Chikso rump during 28 days of aging
ab-23-0001-Supplementary-Table-1.pdf
Supplementary Table S2. Volatile flavor compounds (area unit ├Ś106) in Hanwoo and Chikso loin during 28 days of aging
ab-23-0001-Supplementary-Table-2.pdf
Supplementary Table S3. Pearson correlation coefficient (n = 30) between meat quality traits and metabolites in Hanwoo and Chikso beef cuts
ab-23-0001-Supplementary-Table-3.pdf
Supplementary Table S4. Pearson correlation coefficient (n = 24) between metabolites of Hanwoo and Chikso beef cuts and their sensor values of electronic tongue
ab-23-0001-Supplementary-Table-4.pdf
Figure┬Ā1
The pH (a), CIE L* (b), CIE a* (c), and CIE b* (d) values of Hanwoo and Chikso beef cuts during the wet aging period. CIE, Commission Internationale dŌĆÖEclairage. aŌĆōc Different letters within the same breed indicate significant differences (p<0.05). x,y Different letters within the same wet aging period indicate significant differences (p<0.05).
Figure┬Ā2
The shear force values (a) and MFI (b) of Hanwoo and Chikso beef cuts during the wet aging period. MFI, myofibrillar fragmentation index. aŌĆōc Different letters within the same breed indicate significant differences (p<0.05). x,y Different letters within the same wet aging period indicate significant differences (p<0.05).
Figure┬Ā3
Multivariate analyses and variable importance in projection (VIP) for each analysis for the discrimination of metabolite profile between Hanwoo and Chikso beef during wet aging. Orthogonal partial least squares-discriminant analysis (OPLS-DA) between Hanwoo and Chikso beef for whole wet aging period (days 0 to 28) (a) and partial least squares-discriminant analysis (PLS-DA) between Hanwoo and Chikso rump at each wet aging period (b). Multivariate analyses were performed on beef loins in the same way as beef rumps (c-d). Black arrows in the PLS-DA plot illustrate the direction of metabolomic change of Hanwoo and Chikso beef during the wet aging period. The red-blue color system was used to represent the relative abundance of each metabolite in the beef sample. The numbers written in each class indicate the wet aging period of beef samples. CL, Chikso loin; CR, Chikso rump; HL, Hanwoo loin; HR, Hanwoo rump. Multivariate analyses and variable importance in projection (VIP) for each analysis for the discrimination of metabolite profile between Hanwoo and Chikso beef during wet aging. Orthogonal partial least squares-discriminant analysis (OPLS-DA) between Hanwoo and Chikso beef for whole wet aging period (days 0 to 28) (a) and partial least squares-discriminant analysis (PLS-DA) between Hanwoo and Chikso rump at each wet aging period (b). Multivariate analyses were performed on beef loins in the same way as beef rumps (cŌĆōd). Black arrows in the PLS-DA plot illustrate the direction of metabolomic change of Hanwoo and Chikso beef during the wet aging period. The red-blue color system was used to represent the relative abundance of each metabolite in the beef sample. The numbers written in each class indicate the wet aging period of beef samples. CL, Chikso loin; CR, Chikso rump; HL, Hanwoo loin; HR, Hanwoo rump.
Figure┬Ā4
Heatmap of the volatile compound profile of Hanwoo and Chikso rump (a) and loin (b) during wet aging. The red-blue color system was used to represent the relative abundance of each volatile organic compound in the beef sample. The numbers written in each class indicate beef samplesŌĆÖ wet aging period (d) of beef samples. CL, Chikso loin; CR, Chikso rump; HL, Hanwoo loin; HR, Hanwoo rump.
Figure┬Ā5
Relative abundances (%) of volatile organic compounds in the rump of Hanwoo (a) and Chikso (b) and the loin of Hanwoo (c) and Chikso (d) during wet aging, respectively. The compounds were assigned to alcohol, aldehyde, ester, fatty acid, furan, hydrocarbon, and ketone according to their functional groups.
Figure┬Ā6
Multivariate analyses and variable importance in projection for each investigation for discriminating volatile organic compound (VOC) profiles between Hanwoo and Chikso beef during wet aging. Orthogonal partial least squares-discriminant analysis (OPLS-DA) between the VOCs in Hanwoo and Chikso beef for the whole wet aging period (days 0 to 28) (a) and partial least squares-discriminant analysis (PLS-DA) between the VOCs in Hanwoo and Chikso rump at each wet aging period (b). Multivariate analyses were performed on beef loins in the same way as beef rumps (cŌĆōd). Black arrows in the PLS-DA plot illustrate the direction of aroma pattern change of Hanwoo and Chikso beef during wet aging period. The red-blue color system was used to represent the relative abundance of each VOC in the beef sample. The numbers written in each class indicate the wet aging period of beef samples. CL, Chikso loin; CR, Chikso rump; HL, Hanwoo loin; HR, Hanwoo rump. Multivariate analyses and variable importance in projection for each investigation for discriminating volatile organic compound (VOC) profiles between Hanwoo and Chikso beef during wet aging. Orthogonal partial least squares-discriminant analysis (OPLS-DA) between the VOCs in Hanwoo and Chikso beef for the whole wet aging period (days 0 to 28) (a) and partial least squares-discriminant analysis (PLS-DA) between the VOCs in Hanwoo and Chikso rump at each wet aging period (b). Multivariate analyses were performed on beef loins in the same way as beef rumps (cŌĆōd). Black arrows in the PLS-DA plot illustrate the direction of aroma pattern change of Hanwoo and Chikso beef during wet aging period. The red-blue color system was used to represent the relative abundance of each VOC in the beef sample. The numbers written in each class indicate the wet aging period of beef samples. CL, Chikso loin; CR, Chikso rump; HL, Hanwoo loin; HR, Hanwoo rump.
Figure┬Ā7
Radar plot and principal component analysis according to the relative taste intensity between Hanwoo and Chikso rump (a) and loin (b) during wet aging. The taste intensity was evaluated using an electronic tongue with seven sensors, where AHS, CTS, NMS, ANS, and SCS respond to sour, salty, umami, sweet, and bitterness, respectively, while PKS and CPS represent universal taste intensity. The numbers written in each class indicate the wet aging period (d) of beef samples. C, Chikso; CL, Chikso loin; CR, Chikso rump; H, Hanwoo; HL, Hanwoo loin; HR, Hanwoo rump.
Table┬Ā1
Changes in the free amino acids, dipeptides and derivatives contents (mg/100 g meat) in Hanwoo and Chikso rump during 28 days of wet aging
| Item | Breed | Aging period (d) | SEM1) | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 0 | 7 | 14 | 21 | 28 | |||
| Alanine | Hanwoo | 20.19c | 21.16cy | 27.93b | 23.57bcy | 36.35a | 1.391 |
| Chikso | 21.16c | 25.66bcx | 33.85ab | 32.87abx | 42.44a | 2.249 | |
| SEM2) | 1.054 | 0.675 | 1.720 | 2.308 | 2.762 | ||
| Asparagine | Hanwoo | 2.78c | 3.77cy | 6.55by | 6.52by | 9.13ay | 0.282 |
| Chikso | 2.68c | 5.09bcx | 9.59bx | 9.96bx | 16.14ax | 1.137 | |
| SEM2) | 0.376 | 0.250 | 0.296 | 0.614 | 1.661 | ||
| Glutamate | Hanwoo | 6.97d | 8.60cdy | 12.15bcy | 12.95by | 17.88ay | 0.815 |
| Chikso | 5.79c | 10.83cx | 16.56bx | 21.10bx | 34.60ax | 1.138 | |
| SEM2) | 0.359 | 0.445 | 0.617 | 1.179 | 1.673 | ||
| Glycine | Hanwoo | 12.28b | 13.53by | 18.50ax | 11.71b | 11.72by | 0.791 |
| Chikso | 13.01b | 14.63bx | 11.78by | 12.62b | 21.84ax | 0.635 | |
| SEM2) | 0.925 | 0.244 | 0.574 | 0.746 | 0.878 | ||
| Isoleucine | Hanwoo | 2.37d | 4.53cdy | 9.17aby | 8.55bx | 10.94ay | 0.398 |
| Chikso | 2.62d | 6.87cdx | 11.23bcx | 14.41bx | 25.22ax | 1.374 | |
| SEM2) | 0.175 | 0.163 | 0.447 | 1.101 | 1.910 | ||
| Leucine | Hanwoo | 4.34d | 8.31cy | 14.80by | 15.28by | 19.52ay | 0.594 |
| Chikso | 4.68e | 13.19dx | 21.50cx | 28.11bx | 45.17ax | 1.358 | |
| SEM2) | 0.247 | 0.176 | 0.483 | 1.812 | 1.374 | ||
| Methionine | Hanwoo | 6.27c | 8.45bcy | 10.93by | 10.65by | 14.11ay | 0.563 |
| Chikso | 6.96d | 10.53c | 14.17bx | 17.03bx | 23.23ax | 0.763 | |
| SEM2) | 0.569 | 0.368 | 0.685 | 0.800 | 0.825 | ||
| Phenylalanine | Hanwoo | 3.15d | 5.62cy | 9.67by | 10.79by | 13.08ay | 0.460 |
| Chikso | 3.45e | 8.08dx | 12.14cx | 16.69bx | 23.83ax | 0.757 | |
| SEM2) | 0.174 | 0.180 | 0.465 | 1.121 | 0.651 | ||
| Taurine | Hanwoo | 27.69ab | 25.23ab | 21.83ab | 20.62b | 31.23a | 2.087 |
| Chikso | 24.28 | 23.98 | 24.84 | 21.42 | 30.88 | 2.124 | |
| SEM2) | 2.036 | 0.806 | 1.351 | 2.564 | 2.995 | ||
| Tyrosine | Hanwoo | 3.39y | 5.85y | 12.88 | 3.55y | 6.02y | 3.628 |
| Chikso | 4.73dx | 8.99cdx | 12.98bc | 16.82bx | 24.79ax | 1.091 | |
| SEM2) | 0.320 | 0.183 | 5.740 | 0.939 | 1.386 | ||
| Valine | Hanwoo | 3.67d | 5.85cy | 9.82by | 9.93by | 13.96ay | 0.467 |
| Chikso | 3.89d | 8.79cdx | 14.19bcx | 18.99abx | 23.60ax | 1.430 | |
| SEM2) | 0.200 | 0.103 | 0.736 | 1.271 | 1.856 | ||
| Anserine | Hanwoo | 67.55x | 76.90 | 84.78 | 78.49 | 68.61y | 5.883 |
| Chikso | 56.78by | 72.34ab | 70.63ab | 73.86a | 85.33ax | 3.543 | |
| SEM2) | 2.484 | 3.821 | 5.874 | 7.249 | 3.175 | ||
| Carnosine | Hanwoo | 193.23ab | 223.95abx | 192.54ab | 239.87a | 163.67by | 14.781 |
| Chikso | 145.13c | 175.81bcy | 163.95c | 228.56a | 213.19abx | 10.537 | |
| SEM2) | 16.111 | 9.189 | 11.695 | 15.231 | 10.534 | ||
| Creatine | Hanwoo | 274.53 | 258.39 | 305.93 | 245.72 | 278.06 | 14.346 |
| Chikso | 288.17 | 278.28 | 302.93 | 273.76 | 312.95 | 12.322 | |
| SEM2) | 14.684 | 6.068 | 15.437 | 13.073 | 15.246 | ||
| L-Carnitine | Hanwoo | 52.41bcy | 43.47cy | 69.30aby | 50.70bc | 72.78a | 4.034 |
| Chikso | 75.14abcx | 74.52bcx | 106.29ax | 57.75c | 95.23ab | 6.799 | |
| SEM2) | 3.979 | 1.788 | 6.919 | 5.236 | 7.870 | ||
| N,N-Dimethylglycine | Hanwoo | 0.69b | 0.64b | 0.86a | 0.67b | 0.79ab | 0.035 |
| Chikso | 0.71 | 0.69 | 0.84 | 0.75 | 0.94 | 0.080 | |
| SEM2) | 0.035 | 0.013 | 0.036 | 0.044 | 0.119 | ||
| o-acetylcarnitine | Hanwoo | 26.06y | 21.43y | 22.68x | 21.86 | 24.44x | 1.396 |
| Chikso | 36.08ax | 29.35ax | 5.14cy | 21.17b | 5.32cy | 1.488 | |
| SEM2) | 2.108 | 1.418 | 0.404 | 1.499 | 1.243 | ||
| Total | Hanwoo | 707.58 | 735.68 | 830.32 | 771.42 | 792.29y | 38.019 |
| Chikso | 695.24b | 767.65b | 832.60b | 865.88ab | 1,024.69ax | 38.448 | |
| SEM2) | 34.460 | 14.599 | 39.126 | 49.877 | 43.476 | ||
Table┬Ā2
Changes in the nucleotides and nucleosides, organic acids, ethanol and niacinamide contents (mg/100 g meat) in Hanwoo and Chikso rump during 28 days of wet aging
| Item | Breed | Aging period (d) | SEM1) | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 0 | 7 | 14 | 21 | 28 | |||
| Nucleotide and nucleosides | |||||||
| ŌĆāHypoxanthine | Hanwoo | 21.94c | 32.69bc | 37.03ab | 41.45ab | 48.59a | 2.566 |
| Chikso | 17.83c | 29.79b | 42.88a | 37.73ab | 42.22a | 2.316 | |
| SEM2) | 1.862 | 2.664 | 2.099 | 2.440 | 2.992 | ||
| ŌĆāIMP | Hanwoo | 71.08a | 48.22b | 49.99b | 32.41bc | 17.99cy | 4.457 |
| Chikso | 83.54a | 58.33b | 45.44c | 30.58d | 30.06dx | 1.825 | |
| SEM2) | 3.854 | 5.789 | 1.876 | 1.961 | 1.505 | ||
| ŌĆāInosine | Hanwoo | 14.56ab | 14.81aby | 17.78a | 13.21b | 11.56by | 0.862 |
| Chikso | 16.49b | 19.07abx | 18.19ab | 17.67ab | 19.68ax | 0.662 | |
| SEM2) | 0.630 | 0.611 | 0.726 | 1.229 | 0.384 | ||
| ŌĆāUridine | Hanwoo | 1.26c | 1.54bc | 2.14ab | 1.82ab | 1.99ay | 0.088 |
| Chikso | 1.05c | 1.46bc | 2.05ab | 2.54a | 2.70ax | 0.181 | |
| SEM2) | 0.083 | 0.044 | 0.147 | 0.241 | 0.113 | ||
| ŌĆāSubtotal | Hanwoo | 107.57a | 95.72ab | 104.81a | 87.08ab | 78.13bc | 4.805 |
| Chikso | 118.17a | 107.18ab | 106.51ab | 85.98c | 91.96bc | 4.124 | |
| SEM2) | 5.586 | 3.906 | 3.789 | 5.066 | 3.709 | ||
| Organic acids | |||||||
| ŌĆāAcetate | Hanwoo | 4.80c | 5.40c | 9.45by | 8.22b | 16.87a | 0.530 |
| Chikso | 4.49b | 5.93b | 16.82ax | 6.78b | 20.89a | 0.896 | |
| SEM2) | 0.276 | 0.315 | 0.242 | 0.533 | 1.480 | ||
| ŌĆāFormate | Hanwoo | 0.31c | 0.33c | 0.46cy | 2.02bx | 3.95ax | 0.233 |
| Chikso | 0.28c | 0.32c | 1.32bx | 0.34cy | 1.90ay | 0.099 | |
| SEM2) | 0.033 | 0.014 | 0.101 | 0.058 | 0.381 | ||
| ŌĆāFumarate | Hanwoo | 1.61by | 2.73ab | 6.38a | 3.61aby | 6.56a | 0.840 |
| Chikso | 3.23x | 3.99 | 5.87 | 5.29x | 6.57 | 0.798 | |
| SEM2) | 0.186 | 1.381 | 0.457 | 0.377 | 1.031 | ||
| ŌĆāLactate | Hanwoo | 476.11b | 454.23by | 593.35a | 454.06b | 539.37aby | 21.588 |
| Chikso | 506.60bc | 524.81bcx | 591.92ab | 493.73c | 620.41ax | 20.118 | |
| SEM2) | 19.887 | 13.668 | 20.418 | 27.773 | 20.159 | ||
| ŌĆāSubtotal | Hanwoo | 482.82bc | 462.69cy | 609.64a | 467.91bc | 566.75ab | 21.933 |
| Chikso | 514.60c | 535.04bcx | 615.92ab | 506.15c | 649.77a | 21.008 | |
| SEM2) | 20.101 | 14.669 | 20.315 | 28.472 | 21.527 | ||
| Others | |||||||
| ŌĆāEthanol | Hanwoo | 0.56y | 0.56y | 0.93 | 1.15 | 1.88 | 0.311 |
| Chikso | 1.96ax | 1.05bcx | 0.95c | 1.72ab | 1.96a | 0.155 | |
| SEM2) | 0.163 | 0.084 | 0.133 | 0.157 | 0.476 | ||
| ŌĆāNiacinamide | Hanwoo | 3.37b | 3.68aby | 4.32a | 3.72ab | 3.47aby | 0.189 |
| Chikso | 3.41b | 4.39abx | 4.63a | 4.76a | 5.31ax | 0.242 | |
| SEM2) | 0.154 | 0.160 | 0.146 | 0.284 | 0.292 | ||
Table┬Ā3
Changes in the free amino acids, dipeptides and derivatives contents (mg/100 g meat) in Hanwoo and Chikso loin during 28 days of wet aging
| Item | Breed | Aging period (d) | SEM1) | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 0 | 7 | 14 | 21 | 28 | |||
| Alanine | Hanwoo | 19.07c | 21.34bcy | 27.31abc | 29.55aby | 36.89a | 2.110 |
| Chikso | 22.80c | 28.33bcx | 28.12bc | 37.09ax | 33.37ab | 1.490 | |
| SEM2) | 1.681 | 0.695 | 1.095 | 1.113 | 3.306 | ||
| Asparagine | Hanwoo | 2.70c | 2.83y | 4.37bc | 5.48b | 8.73a | 0.433 |
| Chikso | 3.10d | 4.91cdx | 5.37bc | 7.55ab | 8.46a | 0.486 | |
| SEM2) | 0.374 | 0.132 | 0.405 | 0.663 | 0.547 | ||
| Glutamate | Hanwoo | 7.73c | 7.82c | 11.04bc | 11.87by | 17.05a | 0.745 |
| Chikso | 6.50c | 8.89bc | 10.95b | 16.31ax | 17.08a | 0.861 | |
| SEM2) | 0.753 | 0.741 | 0.425 | 1.123 | 0.827 | ||
| Glycine | Hanwoo | 9.58 | 10.12 | 10.13 | 10.95 | 10.79 | 1.006 |
| Chikso | 9.06 | 9.26 | 10.85 | 10.82 | 7.32 | 0.870 | |
| SEM2) | 0.610 | 0.790 | 0.878 | 0.866 | 1.381 | ||
| Isoleucine | Hanwoo | 1.77d | 2.45cdy | 4.17bc | 5.56ab | 7.21a | 0.388 |
| Chikso | 1.85c | 4.37bcx | 4.80b | 8.12a | 7.86a | 0.620 | |
| SEM2) | 0.203 | 0.130 | 0.377 | 0.708 | 0.797 | ||
| Leucine | Hanwoo | 3.21c | 4.59cy | 7.75b | 10.11a | 12.22a | 0.485 |
| Chikso | 3.27b | 7.89bx | 9.03ab | 14.47a | 14.75a | 1.245 | |
| SEM2) | 0.372 | 0.238 | 0.369 | 1.269 | 1.590 | ||
| Methionine | Hanwoo | 5.62b | 6.16b | 8.95b | 8.07b | 13.93a | 1.048 |
| Chikso | 7.23 | 8.36 | 8.75 | 12.22 | 11.12 | 1.533 | |
| SEM2) | 0.618 | 0.966 | 1.806 | 1.660 | 1.137 | ||
| Phenylalanine | Hanwoo | 1.96c | 2.85cy | 4.88b | 6.59a | 7.70a | 0.264 |
| Chikso | 2.04b | 5.18bx | 5.56b | 9.73a | 9.65a | 0.767 | |
| SEM2) | 0.214 | 0.167 | 0.220 | 0.835 | 0.909 | ||
| Taurine | Hanwoo | 25.39 | 27.21 | 28.21 | 31.48x | 19.18 | 3.098 |
| Chikso | 24.43 | 28.61 | 21.93 | 23.83y | 26.96 | 2.337 | |
| SEM2) | 3.918 | 3.013 | 2.072 | 0.829 | 2.868 | ||
| Tyrosine | Hanwoo | 2.17 | 3.09y | 2.59 | 2.35y | 2.39y | 0.229 |
| Chikso | 2.10d | 5.05bcx | 3.84cd | 5.94bx | 9.58ax | 0.446 | |
| SEM2) | 0.240 | 0.243 | 0.454 | 0.520 | 0.191 | ||
| Valine | Hanwoo | 2.76d | 3.59dy | 5.74c | 7.82b | 11.66a | 0.344 |
| Chikso | 2.72c | 5.93cx | 7.31bc | 11.18ab | 14.05a | 1.124 | |
| SEM2) | 0.278 | 0.080 | 0.497 | 0.982 | 1.469 | ||
| Anserine | Hanwoo | 30.78 | 23.46y | 40.52x | 44.98x | 27.01 | 4.681 |
| Chikso | 38.42 | 37.51x | 27.26y | 32.64y | 21.38 | 3.815 | |
| SEM2) | 7.514 | 2.737 | 3.275 | 3.023 | 2.708 | ||
| Carnosine | Hanwoo | 113.62 | 82.50y | 125.19 | 109.39 | 102.65 | 14.552 |
| Chikso | 140.56 | 157.95x | 93.98 | 127.33 | 98.79 | 14.353 | |
| SEM2) | 15.561 | 9.499 | 11.573 | 19.591 | 13.939 | ||
| Creatine | Hanwoo | 196.29 | 183.25y | 215.77x | 194.61 | 180.51 | 15.926 |
| Chikso | 212.32abc | 241.85ax | 171.57bcy | 218.37ab | 152.20c | 13.072 | |
| SEM2) | 18.660 | 3.076 | 8.879 | 12.089 | 21.879 | ||
| L-Carnitine | Hanwoo | 48.35 | 49.04y | 62.74y | 51.19y | 64.48 | 5.276 |
| Chikso | 74.16 | 71.68x | 90.08x | 82.25x | 86.03 | 4.871 | |
| SEM2) | 7.126 | 2.893 | 2.418 | 4.325 | 6.724 | ||
| N,N-Dimethylglycine | Hanwoo | 0.53 | 0.49y | 0.63x | 0.56 | 0.67 | 0.041 |
| Chikso | 0.55 | 0.62x | 0.50y | 0.66 | 0.63 | 0.042 | |
| SEM2) | 0.059 | 0.014 | 0.026 | 0.024 | 0.061 | ||
| o-acetylcarnitine | Hanwoo | 20.37a | 20.73ay | 22.47ax | 21.93a | 2.01by | 1.990 |
| Chikso | 24.32a | 30.07ax | 3.19by | 29.89a | 3.43bx | 2.317 | |
| SEM2) | 2.885 | 1.860 | 2.211 | 2.556 | 0.338 | ||
| Total | Hanwoo | 491.89 | 451.52y | 582.46 | 552.49 | 525.08 | 40.922 |
| Chikso | 575.43 | 656.44x | 503.08 | 648.41 | 522.66 | 35.414 | |
| SEM2) | 53.399 | 5.492 | 21.354 | 35.547 | 52.160 | ||
Table┬Ā4
Changes in the nucleotides and nucleosides, organic acids, ethanol and niacinamide contents (mg/100 g meat) in Hanwoo and Chikso loin during 28 days of wet aging
| Item | Breed | Aging period (d) | SEM1) | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 0 | 7 | 14 | 21 | 28 | |||
| Nucleotide and nucleosides | |||||||
| ŌĆāHypoxanthine | Hanwoo | 17.25b | 23.75by | 33.39ax | 35.48a | 37.57a | 1.778 |
| Chikso | 15.76c | 28.64bx | 23.29bcy | 39.39a | 26.98b | 1.978 | |
| SEM2) | 1.428 | 0.318 | 1.124 | 1.137 | 3.604 | ||
| ŌĆāIMP | Hanwoo | 32.33a | 13.15by | 7.67b | 5.47b | 4.54b | 2.752 |
| Chikso | 49.42a | 38.33ax | 6.90b | 9.29b | 4.56b | 3.359 | |
| SEM2) | 4.685 | 4.161 | 0.920 | 2.601 | 0.527 | ||
| ŌĆāInosine | Hanwoo | 8.87a | 7.02ay | 7.50ax | 6.27a | 1.46by | 0.649 |
| Chikso | 9.84a | 10.32ax | 4.08by | 5.59b | 3.89bx | 0.824 | |
| SEM2) | 1.098 | 0.674 | 0.387 | 0.889 | 0.387 | ||
| ŌĆāUridine | Hanwoo | 1.13 | 1.26y | 1.46 | 1.40 | 1.87 | 0.227 |
| Chikso | 0.88b | 1.52ax | 1.19ab | 1.28ab | 1.25ab | 0.122 | |
| SEM2) | 0.108 | 0.055 | 0.075 | 0.088 | 0.372 | ||
| ŌĆāSubtotal | Hanwoo | 58.45 | 43.92y | 48.56x | 47.21 | 43.58 | 4.147 |
| Chikso | 75.03a | 77.29ax | 34.27by | 54.28ab | 35.43b | 5.085 | |
| SEM2) | 6.929 | 4.857 | 1.558 | 4.601 | 3.528 | ||
| Organic acids | |||||||
| ŌĆāAcetate | Hanwoo | 3.70b | 4.07b | 6.89by | 12.43b | 29.85a | 1.887 |
| Chikso | 4.31c | 4.07c | 12.87bx | 14.87b | 21.95a | 1.305 | |
| SEM2) | 0.743 | 0.272 | 0.548 | 0.736 | 3.419 | ||
| ŌĆāFormate | Hanwoo | 0.26c | 0.24cy | 2.76c | 8.19b | 17.73ax | 1.056 |
| Chikso | 0.31b | 0.32bx | 3.84b | 12.71a | 8.33ay | 0.956 | |
| SEM2) | 0.044 | 0.015 | 0.432 | 1.743 | 1.360 | ||
| ŌĆāFumarate | Hanwoo | 0.61by | 1.38b | 1.22b | 4.24a | 4.92a | 0.513 |
| Chikso | 0.99bx | 1.29b | 1.33b | 3.69a | 3.34a | 0.197 | |
| SEM2) | 0.047 | 0.396 | 0.130 | 0.388 | 0.655 | ||
| ŌĆāLactate | Hanwoo | 313.32 | 302.02y | 345.15x | 357.84 | 350.02 | 29.294 |
| Chikso | 337.30 | 385.98x | 288.36y | 366.71 | 328.70 | 22.912 | |
| SEM2) | 18.764 | 17.058 | 9.331 | 12.308 | 50.756 | ||
| ŌĆāSubtotal | Hanwoo | 317.89 | 307.72y | 356.02x | 382.70 | 402.52 | 32.079 |
| Chikso | 342.92 | 391.67x | 306.39y | 397.98 | 362.32 | 24.079 | |
| SEM2) | 19.167 | 17.482 | 9.880 | 12.774 | 55.573 | ||
| Others | |||||||
| ŌĆāEthanol | Hanwoo | 0.52by | 0.39b | 0.49b | 0.86b | 1.51ax | 0.134 |
| Chikso | 1.00x | 0.57 | 0.55 | 1.07 | 0.69y | 0.122 | |
| SEM2) | 0.088 | 0.047 | 0.089 | 0.167 | 0.191 | ||
| ŌĆāNiacinamide | Hanwoo | 2.06a | 2.29ay | 2.70a | 2.20a | 0.65b | 0.198 |
| Chikso | 2.77ab | 3.43ax | 2.09bc | 2.34b | 1.34c | 0.187 | |
| SEM2) | 0.188 | 0.162 | 0.159 | 0.215 | 0.230 | ||
REFERENCES
1. Suh S, Kim YS, Cho CY, et al. Assessment of genetic diversity, relationships and structure among Korean native cattle breeds using microsatellite markers. Asian-Australas J Anim Sci 2014; 27:1548ŌĆō53. https://doi.org/10.5713/ajas.2014.14435



2. Song JS, Seong HS, Choi BH, et al. Genome-wide analysis of Hanwoo and Chikso populations using the BovineSNP50 genotyping array. Genes Genomics 2018; 40:1373ŌĆō82. https://doi.org/10.1007/s13258-018-0733-x


3. Piao MY, Yong HI, Lee HJ, et al. Comparison of fatty acid profiles and volatile compounds among quality grades and their association with carcass characteristics in longissimus dorsi and semimembranosus muscles of Korean cattle steer. Livest Sci 2017; 198:147ŌĆō56. https://doi.org/10.1016/j.livsci.2017.02.021

4. Utama DT, Lee CW, Park YS, Jang A, Lee SK. Comparison of meat quality, fatty acid composition and aroma volatiles of Chikso and Hanwoo beef. Asian-Australas J Anim Sci 2018; 31:1500ŌĆō6. https://doi.org/10.5713/ajas.17.0902



5. Lee T, Joo N. Comparison of fatty acid, amino acid, and sensory properties of Chikhanwoo (Korean brindle cattle) and Hanwoo (Korean native cattle). Culin Sci Hosp Res 2022; 28:18ŌĆō27. https://doi.org/10.20878/cshr.2022.28.2.003

6. Wei M, Liu X, Xie P, et al. Characterization of volatile profiles and correlated contributing compounds in pan-fried steaks from different Chinese yellow cattle breeds through GC-Q-orbitrap, e-nose, and sensory evaluation. Molecules 2022; 27:3593https://doi.org/10.3390/molecules27113593



7. Van Ba H, Oliveros CM, Park K, Dashdorj D, Hwang I. Effect of marbling and chilled ageing on meat-quality traits, volatile compounds and sensory characteristics of beef longissimus dorsi muscle. Anim Prod Sci 2017; 57:981ŌĆō92. https://doi.org/10.1071/AN15676

8. Xu L, Liu S, Cheng Y, Qian H. The effect of aging on beef taste, aroma and texture, and the role of microorganisms: a review. Crit Rev Food Sci Nutr 2023; 63:2129ŌĆō40. https://doi.org/10.1080/10408398.2021.1971156


9. Kodani Y, Miyakawa T, Komatsu T, Tanokura M. NMR-based metabolomics for simultaneously evaluating multiple determinants of primary beef quality in Japanese Black cattle. Sci Rep 2017; 7:1297https://doi.org/10.1038/s41598-017-01272-8



10. Setyabrata D, Cooper BR, Sobreira TJ, Legako JF, Martini S, Kim YHB. Elucidating mechanisms involved in flavor generation of dry-aged beef loins using metabolomics approach. Food Res Int 2021; 139:109969https://doi.org/10.1016/j.foodres.2020.109969


11. Lee D, Lee HJ, Yoon JW, Kim M, Jo C. Effect of different aging methods on the formation of aroma volatiles in beef strip loins. Foods 2021; 10:146https://doi.org/10.3390/foods10010146



12. Li S, Xiang C, Ge Y, Liu H, Zhang D, Wang Z. Differences in eating quality and electronic sense of meat samples as a function of goat breed and postmortem rigor state. Food Res Int 2022; 152:110923https://doi.org/10.1016/j.foodres.2021.110923


13. Ramalingam V, Song Z, Hwang I. The potential role of secondary metabolites in modulating the flavor and taste of the meat. Food Res Int 2019; 122:174ŌĆō82. https://doi.org/10.1016/j.foodres.2019.04.007


14. Lee HJ, Choe J, Kim M, et al. Role of moisture evaporation in the taste attributes of dry- and wet-aged beef determined by chemical and electronic tongue analyses. Meat Sci 2019; 151:82ŌĆō8. https://doi.org/10.1016/j.meatsci.2019.02.001


15. ┼Üliwi┼äska M, Wi┼øniewska P, Dymerski T, Namie┼ønik J, Wardencki W. Food analysis using artificial senses. J Agric Food Chem 2014; 62:1423ŌĆō48. https://doi.org/10.1021/jf403215y


16. Lee D, Lee HJ, Yoon JW, Ryu M, Jo C. Effects of cooking conditions on the physicochemical and sensory characteristics of dry- and wet-aged beef. Anim Biosci 2021; 34:1705ŌĆō16. https://doi.org/10.5713/ab.20.0852



17. Kim SY, Yong HI, Nam KC, Jung S, Yim DG, Jo C. Application of high temperature (14┬░C) aging of beef M. semimembranosus with low-dose electron beam and X-ray irradiation. Meat Sci 2018; 136:85ŌĆō92. https://doi.org/10.1016/j.meatsci.2017.10.016


18. Kim HC, Yim DG, Kim JW, Lee D, Jo C. Nuclear magnetic resonance (NMR)-based quantification on flavor-active and bioactive compounds and application for distinguishment of chicken breeds. Food Sci Anim Resour 2021; 41:312ŌĆō23. https://doi.org/10.5851/kosfa.2020.e10



19. Jin SK, Yim DG. Comparison of effects of two aging methods on the physicochemical traits of pork loin. Food Sci Anim Resour 2020; 40:844ŌĆō51. https://doi.org/10.5851/kosfa.2020.e22



20. Jin SK, Yim DG. Influences of aging methods and temperature on meat quality of pork belly from purebred Berkshire and crossbred Landrace├Ś Yorkshire├Ś Duroc (LYD) pigs. Food Sci Anim Resour 2022; 42:398ŌĆō410. https://doi.org/10.5851/kosfa.2022.e7



21. Ma D, Kim YHB, Cooper B, et al. Metabolomics profiling to determine the effect of postmortem aging on color and lipid oxidative stabilities of different bovine muscles. J Agric Food Chem 2017; 65:6708ŌĆō16. https://doi.org/10.1021/acs.jafc.7b02175


22. Suman SP, Hunt MC, Nair MN, Rentfrow G. Improving beef color stability: Practical strategies and underlying mechanisms. Meat Sci 2014; 98:490ŌĆō504. https://doi.org/10.1016/j.meatsci.2014.06.032


23. Edea Z, Kim KS. Assessment of genetic diversity and signatures of selection in Korean cattle populations. J Anim Breed Genom 2019; 3:113ŌĆō23. https://doi.org/10.12972/jabng.20190013

24. Edea Z, Jung KS, Shin SS, Yoo SW, Choi JW, Kim KS. Signatures of positive selection underlying beef production traits in Korean cattle breeds. J Anim Sci Technol 2020; 62:293ŌĆō305. https://doi.org/10.5187/jast.2020.62.3.293



25. Belew JB, Brooks JC, McKenna DR, Savell JW. WarnerŌĆōBratzler shear evaluations of 40 bovine muscles. Meat Sci 2003; 64:507ŌĆō12. https://doi.org/10.1016/S0309-1740(02)00242-5


26. Laville E, Sayd T, Morzel M, et al. Proteome changes during meat aging in tough and tender beef suggest the importance of apoptosis and protein solubility for beef aging and tenderization. J Agric Food Chem 2009; 57:10755ŌĆō64. https://doi.org/10.1021/jf901949r


27. Aroeira CN, Torres Filho RA, Fontes PR, et al. Comparison of different methods for determining the extent of myofibrillar fragmentation of chilled and frozen/thawed beef across postmortem aging periods. Meat Sci 2020; 160:107955https://doi.org/10.1016/j.meatsci.2019.107955


28. Straadt IK, Aaslyng MD, Bertram HC. An NMR-based metabolomics study of pork from different crossbreeds and relation to sensory perception. Meat Sci 2014; 96:719ŌĆō28. https://doi.org/10.1016/j.meatsci.2013.10.006


29. Kim HJ, Jang A. Correlations between the levels of the bioactive compounds and quality traits in beef loin and round during cold storage. Food Control 2021; 120:107491https://doi.org/10.1016/j.foodcont.2020.107491

30. Dashdorj D, Amna T, Hwang I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: an overview. Eur Food Res Technol 2015; 241:157ŌĆō71. https://doi.org/10.1007/s00217-015-2449-3

31. Wang X, Zhu L, Han Y, et al. Analysis of volatile compounds between raw and cooked beef by HS-SPMEŌĆōGCŌĆōMS. J Food Process Preserv 2018; 42:e13503https://doi.org/10.1111/jfpp.13503

32. Du H, Chen Q, Liu Q, Wang Y, Kong B. Evaluation of flavor characteristics of bacon smoked with different woodchips by HS-SPME-GC-MS combined with an electronic tongue and electronic nose. Meat Sci 2021; 182:108626https://doi.org/10.1016/j.meatsci.2021.108626


33. Inagaki S, Amano Y, Kumazawa K. Identification and characterization of volatile components causing the characteristic flavor of wagyu beef (Japanese Black Cattle). J Agric Food Chem 2017; 65:8691ŌĆō5. https://doi.org/10.1021/acs.jafc.7b02843


34. Chen M, Gao X, Pan D, et al. Taste characteristics and umami mechanism of novel umami peptides and umami-enhancing peptides isolated from the hydrolysates of Sanhuang Chicken. Eur Food Res Technol 2021; 247:1633ŌĆō44. https://doi.org/10.1007/s00217-021-03734-w

35. Kim JH, Kim DH, Ji DS, et al. Effect of aging process and time on physicochemical and sensory evaluation of raw beef top round and shank muscles using an electronic tongue. Korean J Food Sci Anim Resour 2017; 37:823ŌĆō32. https://doi.org/10.5851/kosfa.2017.37.6.823









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print





