 |
 |
| Anim Biosci > Volume 34(6); 2021 > Article |
|
Abstract
Objective
Methods
Results
ACKNOWLEDGMENTS
Figure┬Ā1

Figure┬Ā2
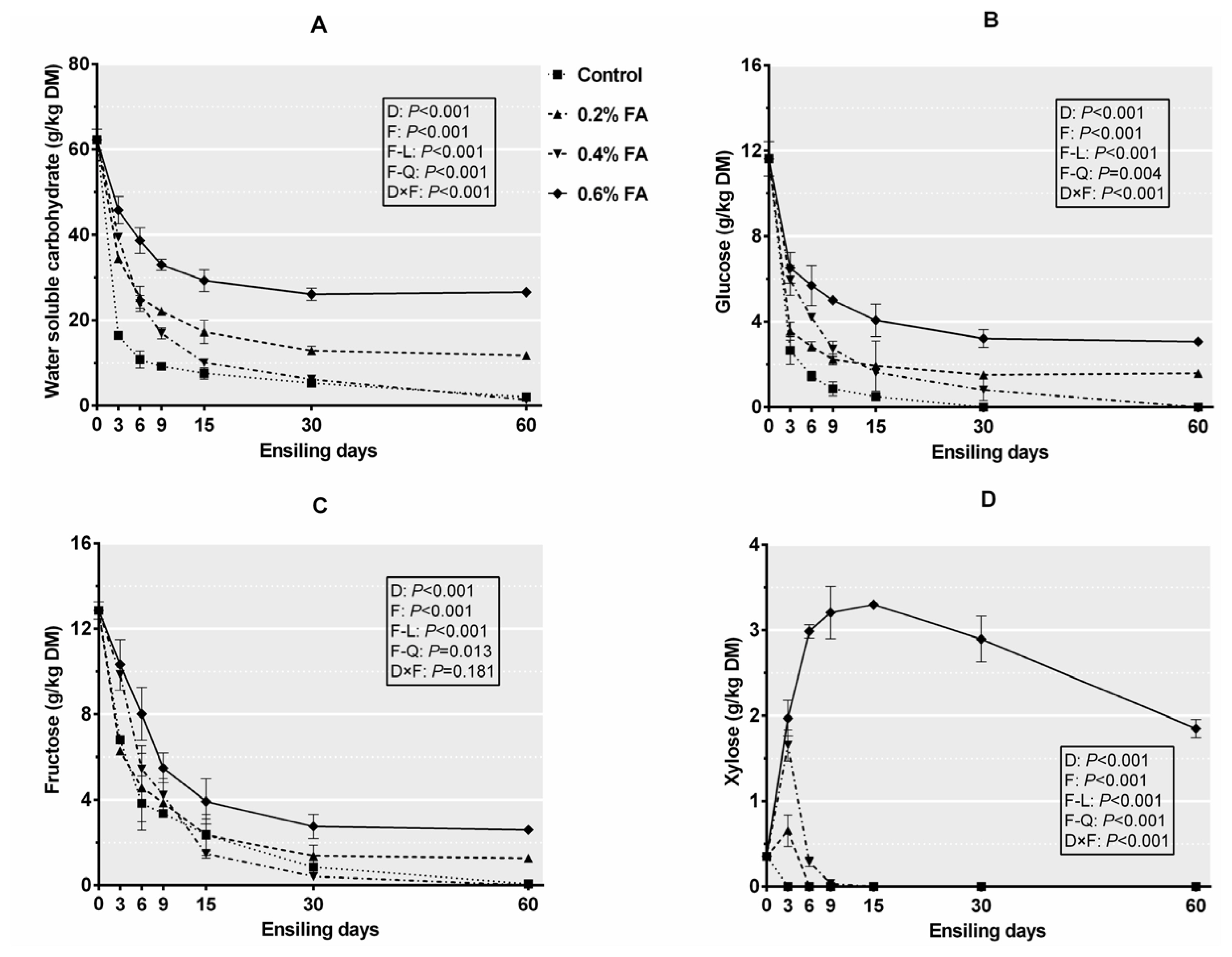
Figure┬Ā3
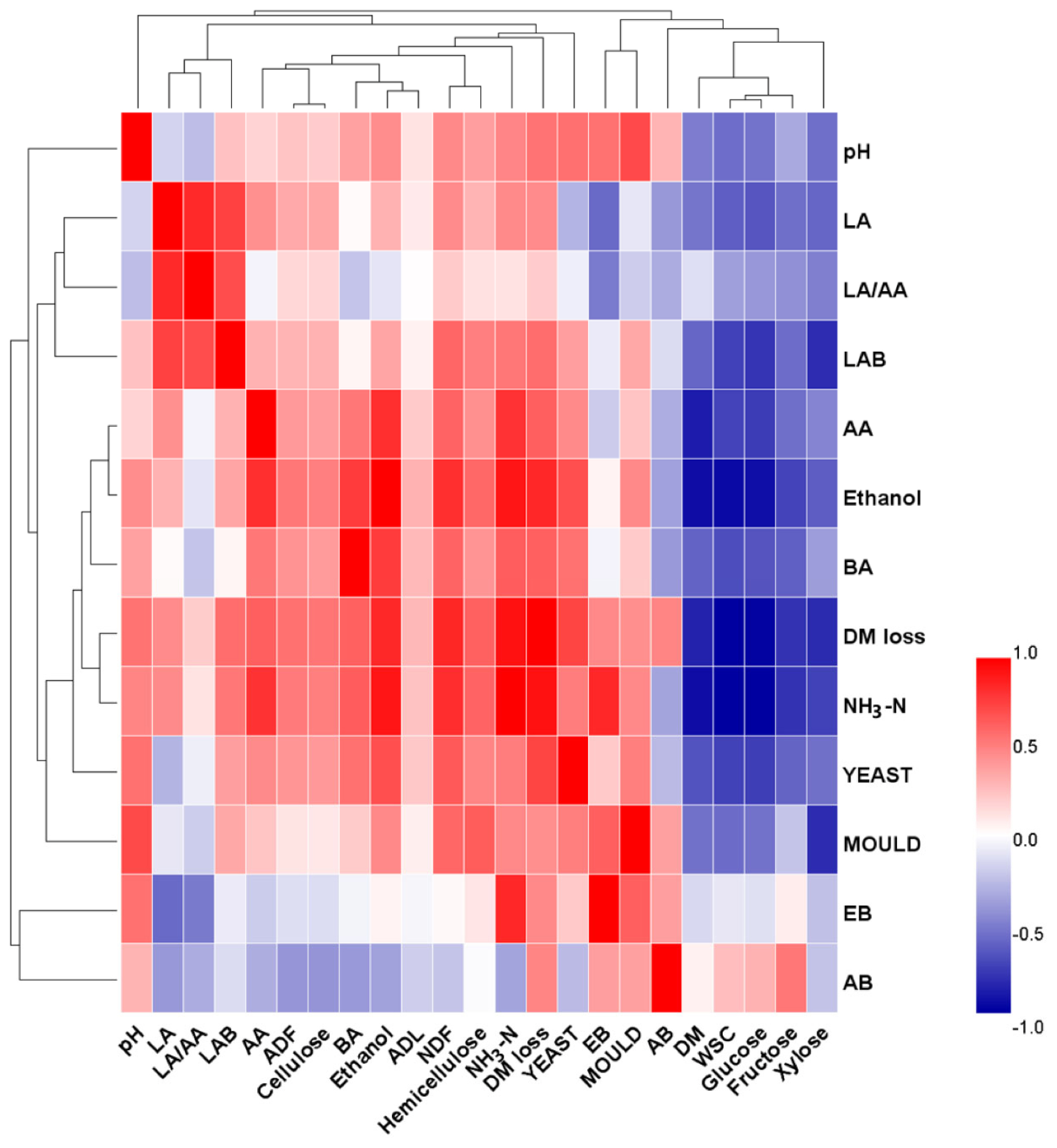
Figure┬Ā4
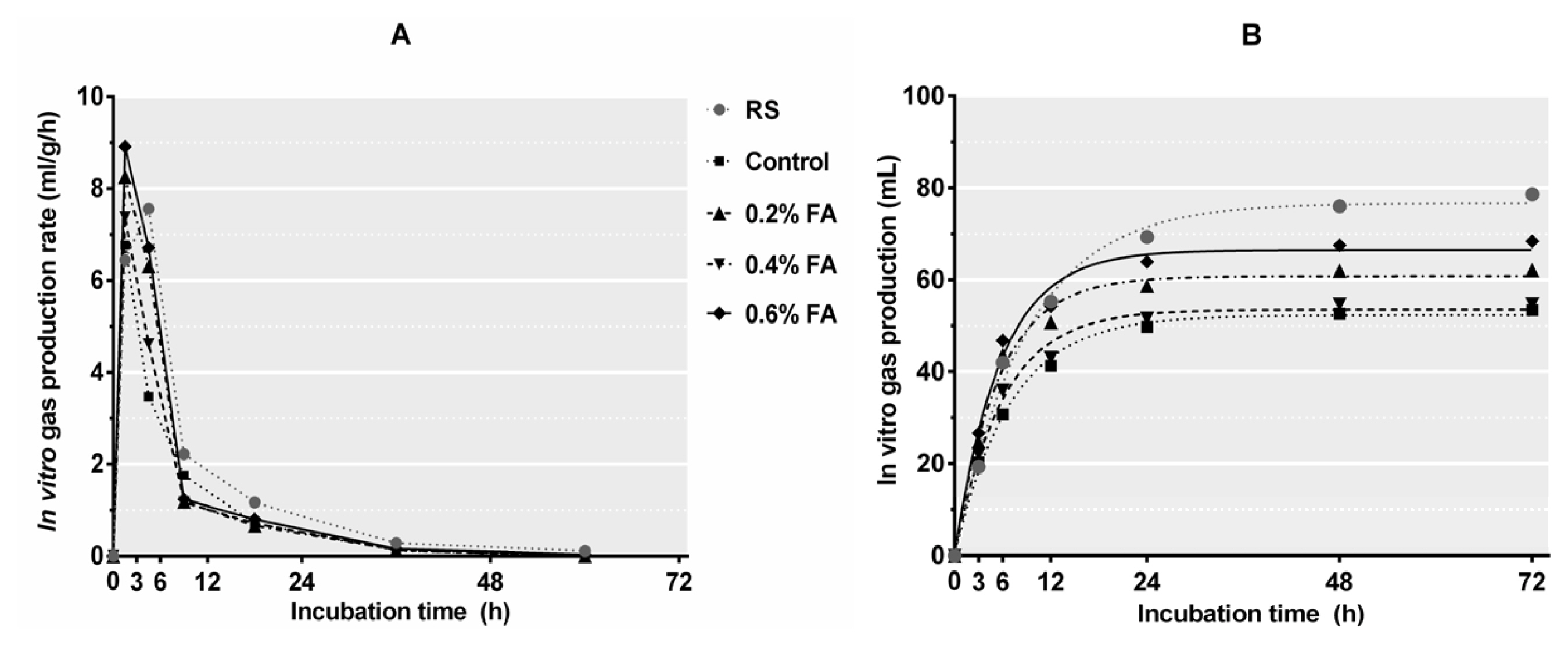
Table┬Ā1
DM, dry matter; FW, fresh weight; CP, crude protein; WSC, water soluble carbohydrate; NDF, neutral detergent fibre; ADF, acid detergent fibre; ADL, acid detergent lignin; BC, buffering capacity; mEq, milligram equivalent; FC, fermentation coefficient; cfu, colony-forming units; LAB, lactic acid bacteria; EB, enterobacteria; AB, aerobic bacteria.
Table┬Ā2
| Items | Treatments1) | Ensiling days | Mean | SEM | p-value2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| 3 | 6 | 9 | 15 | 30 | 60 | D | F | F-L | F-Q | D├ŚF | ||||
| pH | Control | 5.44Aa | 5.23Aab | 4.65b | 4.72ABb | 4.68ABb | 4.78ABb | 4.92A | 0.052 | 0.007 | <0.001 | <0.001 | 0.085 | <0.001 |
| 0.2% FA | 5.06Ba | 5.03Aa | 4.49b | 4.30Cb | 4.19Bb | 4.28Bb | 4.56B | |||||||
| 0.4% FA | 4.32Cc | 4.64Bbc | 4.52bc | 4.80Aabc | 5.18Aab | 5.43Aa | 4.82A | |||||||
| 0.6% FA | 4.03Ca | 4.24Ca | 4.13a | 4.33BCa | 4.28ABa | 4.30Ba | 4.27C | |||||||
| Lactic acid (% DM) | Control | 1.23Ac | 1.92Ac | 2.51Aabc | 3.67Aab | 3.92Aa | 2.28Bbc | 2.59A | 0.210 | <0.001 | <0.001 | <0.001 | 0.020 | <0.001 |
| 0.2% FA | 0.49Bc | 1.18Bbc | 1.81ABbc | 3.49Aab | 5.78Aa | 5.54Aa | 3.05A | |||||||
| 0.4% FA | 0.05Bc | 0.11Cc | 0.27Bbc | 0.63Bab | 0.81Bab | 1.14Ca | 0.50B | |||||||
| 0.6% FA | 0.04Bc | 0.07Cc | 0.16Bbc | 0.11Bbc | 0.42Bab | 0.71Ca | 0.25B | |||||||
| Acetic acid (% DM) | Control | 0.69Ae | 0.83Ade | 1.01Ad | 1.30Ac | 2.24Ab | 2.58Aa | 1.44A | 0.072 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 0.2% FA | 0.30Bc | 0.38Bbc | 0.40Babc | 0.52Babc | 0.60Bab | 0.63Ba | 0.47B | |||||||
| 0.4% FA | 0.23Bd | 0.19Cd | 0.25Ccd | 0.37Bbc | 0.46BCb | 0.74Ba | 0.37C | |||||||
| 0.6% FA | 0.23Bc | 0.20Cc | 0.20Cc | 0.25Bbc | 0.44Ca | 0.38Cab | 0.28D | |||||||
| Butyric acid (% DM) | Control | NDb | NDb | NDb | 0.04b | 0.17Aa | 0.21Aa | 0.07A | 0.010 | <0.001 | <0.001 | <0.001 | 0.028 | <0.001 |
| 0.2% FA | ND | ND | ND | ND | NDB | NDB | 0.00B | |||||||
| 0.4% FA | NDb | NDb | 0.03b | 0.04b | 0.22Aa | 0.27Aa | 0.09A | |||||||
| 0.6% FA | ND | ND | ND | ND | NDB | NDB | 0.00B | |||||||
| LA/AA | Control | 1.77Aab | 2.31Aab | 2.46ABa | 2.84ABa | 1.75Bab | 0.88Cc | 2.00B | 0.032 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 0.2% FA | 1.62Ac | 3.32Abc | 4.43Aabc | 7.44Aabc | 9.57Aa | 8.80Aab | 5.86A | |||||||
| 0.4% FA | 0.20B | 0.57B | 1.08B | 2.01AB | 1.72B | 1.55BC | 1.19BC | |||||||
| 0.6% FA | 0.17Bb | 0.38Bb | 0.80Bb | 0.45Bb | 1.02Bab | 1.90Ba | 0.78C | |||||||
SEM, standard error of means; DM, dry matter; LA/AA, ratio of lactic acid to acetic acid; ND, not detected.
1) Control, no additive; 0.2% FA, 0.2% formic acid; 0.4% FA, 0.4% formic acid; 0.6% FA, 0.6% formic acid;
Table┬Ā3
| Items (log10 cfu/g FW) | Treatments1) | Ensiling days | Mean | SEM | p-value2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| 3 | 6 | 9 | 15 | 30 | 60 | D | F | F-L | F-Q | D├ŚF | ||||
| LAB | Control | 5.64Aabc | 6.23Aa | 5.91Aab | 5.53Bbc | 5.20Bcd | 4.75Bd | 5.54B | 0.158 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 0.2% FA | 4.15Bd | 5.68Bc | 6.09Abc | 6.72Aa | 7.05Aa | 6.13Ab | 5.97A | |||||||
| 0.4% FA | 3.38Cc | 3.43Cc | 3.69Bbc | 4.25Cb | 5.10Ba | 4.93Ba | 4.13C | |||||||
| 0.6% FA | 2.53Dd | 2.74Dcd | 3.13Bbc | 3.33Db | 3.98Ca | 2.90Cbcd | 3.10D | |||||||
| EB | Control | 8.43Aa | 7.70Aab | 7.11Ab | 6.86Ab | 3.89Cc | 3.94Cc | 6.32A | 0.255 | <0.001 | <0.001 | <0.001 | 0.375 | <0.001 |
| 0.2% FA | 7.92Aa | 7.24Ab | 6.81Ac | 6.59Ac | NDDd | NDDd | 4.76B | |||||||
| 0.4% FA | 5.95Bb | 6.10Bab | 6.35Bab | 6.71Aab | 6.99Aa | 6.53Aab | 6.44A | |||||||
| 0.6% FA | 3.61Cc | 3.83Cbc | 3.99Cb | 5.62Ba | 5.69Ba | 5.88Ba | 4.77B | |||||||
| Yeasts | Control | 5.25Aa | 4.86Aab | 4.82Aab | 4.75Aab | 4.23Ab | 4.59Aab | 4.75A | 0.063 | <0.001 | <0.001 | <0.001 | 0.468 | 0.001 |
| 0.2% FA | 3.94Acd | 4.52Aab | 4.76Aa | 4.02Abcd | 4.38Babc | 3.81Bd | 4.24BC | |||||||
| 0.4% FA | 3.89Bc | 3.91Bc | 3.94Bc | 4.56Abc | 5.39Aa | 5.07Aab | 4.46AB | |||||||
| 0.6% FA | 3.78Bb | 3.82Bb | 3.82Bb | 4.09Aab | 4.23Ba | 3.91Bb | 3.94C | |||||||
| Moulds | Control | 4.92A | 4.80A | 4.31A | 3.73A | 3.38A | 3.45A | 4.10A | 0.211 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 0.2% FA | 4.05Ba | 4.12ABa | 3.89Aa | 2.56Bb | NDBc | NDBc | 2.44C | |||||||
| 0.4% FA | 2.48Cc | 3.55Bab | 4.06Aab | 4.32Aa | 4.42Aa | 3.29Abc | 3.69B | |||||||
| 0.6% FA | NDD | NDC | NDB | NDC | NDB | NDB | 0.00D | |||||||
| AB | Control | 7.03Aa | 6.41Ab | NDBc | NDc | NDc | NDc | 2.24A | 0.269 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 0.2% FA | 4.57Cb | 5.02Ba | NDBc | NDc | NDc | NDc | 1.60B | |||||||
| 0.4% FA | 5.31BCa | 4.50Cb | 4.64Ab | NDc | NDc | NDc | 2.41A | |||||||
| 0.6% FA | 3.68Da | NDDb | NDBb | NDb | NDb | NDb | 0.61C | |||||||
cfu, colony-forming units; FW, fresh weight; SEM, standard error of means; LAB, lactic acid bacteria; EB, enterobacteria; ND, not detected; AB, aerobic bacteria.
1) Control, no additive; 0.2% FA, 0.2% formic acid; 0.4% FA, 0.4% formic acid; 0.6% FA, 0.6% formic acid.
Table┬Ā4
| Items | Treatments1) | Ensiling days | Mean | SEM | p-value2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| 3 | 6 | 9 | 15 | 30 | 60 | D | F | F-L | F-Q | D├ŚF | ||||
| DM (% FW) | Control | 35.2Ba | 32.7Bbc | 34.2Bab | 32.8Bbc | 32.0Bbc | 31.8Cc | 33.1C | 0.380 | <0.001 | <0.001 | <0.001 | <0.001 | 0.613 |
| 0.2% FA | 40.0A | 39.3A | 38.9A | 38.1A | 37.7A | 36.8A | 38.5B | |||||||
| 0.4% FA | 39.5Aab | 40.2Aa | 38.6Aabc | 36.9ABabc | 35.7ABbc | 34.5Bc | 37.6B | |||||||
| 0.6% FA | 40.7A | 40.6A | 40.8A | 41.0A | 39.2A | 38.6A | 40.1A | |||||||
| DM loss (% DM) | Control | 8.90Ac | 12.8Ab | 12.4Abc | 14.3Aab | 15.4Aab | 16.8Aa | 13.4A | 0.559 | <0.001 | <0.001 | <0.001 | 0.015 | 0.018 |
| 0.2% FA | 6.70Ab | 8.84ABb | 10.0ABab | 10.7Aa | 10.8ABa | 11.3Ba | 9.72B | |||||||
| 0.4% FA | 3.69Bd | 6.74ABcd | 8.73ABcd | 11.3Abc | 15.7Aab | 17.1Aa | 10.5B | |||||||
| 0.6% FA | 2.81Bc | 3.23Bab | 3.34Bab | 4.20Bab | 5.47Bab | 6.76Ca | 4.30C | |||||||
| NH3-N (% TN) | Control | 9.89Ac | 13.0Abc | 13.8Abc | 14.9Ab | 16.4Aab | 19.8Aa | 14.6A | 0.546 | <0.001 | <0.001 | <0.001 | 0.011 | <0.001 |
| 0.2% FA | 5.43Bb | 6.68Bb | 7.57Bab | 8.78ABa | 9.07Ca | 9.87Ba | 8.57B | |||||||
| 0.4% FA | 3.36Bd | 5.76Bcd | 7.44Bbc | 9.96ABb | 13.9Ba | 18.9Aa | 9.89B | |||||||
| 0.6% FA | 3.18Bc | 3.97Bc | 4.12Cbc | 5.12Babc | 6.39Dab | 7.03Ca | 4.97C | |||||||
| Ethanol (% DM) | Control | 0.82Ad | 1.23Ac | 1.39Abc | 1.63Ab | 2.04Aa | 2.14Aa | 1.54A | 0.065 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 0.2% FA | 0.42Bb | 0.62Bab | 0.70BCab | 0.77Ca | 0.73Ca | 0.61Bab | 0.64C | |||||||
| 0.4% FA | 0.38BCd | 0.60Bcd | 0.82Bbc | 1.09Bb | 1.59Ba | 1.76Aa | 1.04B | |||||||
| 0.6% FA | 0.32Cd | 0.39Bcd | 0.44Cbc | 0.52Cab | 0.59Ca | 0.54Bab | 0.47D | |||||||
SEM, standard error of means; DM, dry matter; FW, fresh weight; NH3-N, ammonia nitrogen; TN, total nitrogen.
1) Control, no additive; 0.2% FA, 0.2% formic acid; 0.4% FA, 0.4% formic acid; 0.6% FA, 0.6% formic acid.
Table┬Ā5
| Items | Treatments1) | Ensiling days | Mean | SEM | p-value2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| 3 | 6 | 9 | 15 | 30 | 60 | D | F | F-L | F-Q | D├ŚF | ||||
| NDF (% DM) | Control | 63.2Ad | 66.1Ac | 68.0Ab | 68.3Ab | 69.2Ab | 71.1Aa | 67.7A | 0.496 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 0.2% FA | 62.0ABb | 64.1Bab | 66.1ABa | 66.0ABa | 65.6Ba | 64.9Bab | 64.8B | |||||||
| 0.4% FA | 61.0Cd | 63.3Bcd | 64.9ABbcd | 66.5Aabc | 68.5Aab | 71.3Aa | 65.9AB | |||||||
| 0.6% FA | 59.8Ca | 58.6Cab | 58.4Cab | 58.7Bab | 58.4Cab | 57.9Cb | 58.6C | |||||||
| ADF (% DM) | Control | 39.0c | 40.4bc | 41.5ab | 41.9ab | 42.0ab | 42.9a | 41.3 | 0.292 | 0.005 | 0.058 | 0.010 | 0.694 | 0.268 |
| 0.2% FA | 38.2 | 39.1 | 40.0 | 40.7 | 41.0 | 41.1 | 40.0 | |||||||
| 0.4% FA | 37.5d | 38.9cd | 39.3bc | 40.0bc | 42.0ab | 43.4a | 40.2 | |||||||
| 0.6% FA | 38.0 | 38.3 | 38.9 | 39.6 | 39.6 | 39.8 | 39.0 | |||||||
| ADL (% DM) | Control | 5.33 | 5.47 | 5.42 | 5.55 | 5.58 | 5.87 | 5.54 | 0.061 | 0.544 | 0.423 | 0.250 | 0.649 | 0.916 |
| 0.2% FA | 5.22 | 5.35 | 5.23 | 5.31 | 5.29 | 5.35 | 5.29 | |||||||
| 0.4% FA | 5.15 | 5.27 | 5.29 | 5.39 | 5.57 | 5.97 | 5.44 | |||||||
| 0.6% FA | 5.11 | 5.18 | 5.22 | 5.33 | 5.29 | 5.36 | 5.25 | |||||||
| Cellulose (% DM) | Control | 33.7b | 34.9ab | 36.1a | 36.3a | 36.5a | 37.0a | 35.8 | 0.265 | 0.007 | 0.072 | 0.013 | 0.727 | 0.131 |
| 0.2% FA | 33.0b | 33.7ab | 34.8ab | 35.4a | 35.7a | 35.7a | 34.7 | |||||||
| 0.4% FA | 32.4b | 33.6ab | 34.0ab | 34.7ab | 36.4a | 37.5a | 34.7 | |||||||
| 0.6% FA | 32.9 | 33.1 | 33.7 | 34.3 | 34.3 | 34.4 | 33.8 | |||||||
| Hemicellulose (% DM) | Control | 24.2Ac | 25.7Abc | 26.5Aab | 26.4Aab | 27.2Aab | 28.2Aa | 26.4A | 0.399 | 0.730 | <0.001 | <0.001 | <0.001 | 0.139 |
| 0.2% FA | 23.8A | 25.1A | 26.1A | 25.3AB | 24.7B | 23.8B | 24.8B | |||||||
| 0.4% FA | 23.5Ac | 24.4Abc | 25.6Aab | 26.4Aab | 26.5Aab | 27.9Aa | 25.7A | |||||||
| 0.6% FA | 21.9Ba | 20.3Bab | 19.5Bab | 19.0Bab | 18.8Cab | 18.1Cb | 19.6C | |||||||
SEM, standard error of means; NDF, neutral detergent fibre; DM, dry matter; ADF, acid detergent fibre; ADL, acid detergent lignin.
1) Control, no additive; 0.2% FA, 0.2% formic acid; 0.4% FA, 0.4% formic acid; 0.6% FA, 0.6% formic acid.
Table┬Ā6
| Items | Treatments1) | SEM | p-value2) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Control | 0.2% FA | 0.4% FA | 0.6% FA | F | F-L | F-Q | ||
| CP (% DM) | 5.89B | 6.27A | 6.05AB | 6.35A | 0.044 | 0.009 | 0.009 | 0.617 |
| Ash (% DM) | 14.9A | 13.3B | 15.0A | 12.8B | 0.318 | 0.001 | 0.007 | 0.335 |
| Potential gas production, b (mL) | 52.3C | 60.8B | 53.5C | 66.5A | 1.473 | <0.001 | <0.001 | 0.034 |
| Gas production rate constant, c (mL/h) | 0.15 | 0.18 | 0.17 | 0.18 | 0.012 | 0.052 | 0.035 | 0.195 |
| In vitro dry matter degradability (%) | 53.5 | 52.5 | 51.0 | 53.6 | 1.221 | 0.279 | 0.801 | 0.106 |
| In vitro neutral detergent fibre degradability (%) | 51.4 | 49.5 | 50.7 | 49.0 | 2.430 | 0.852 | 0.755 | 0.787 |
| In vitro acid detergent fibre degradability (%) | 44.5 | 45.3 | 44.8 | 45.6 | 2.838 | 0.725 | 0.418 | 0.701 |






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





