 |
 |
| Anim Biosci > Volume 33(8); 2020 > Article |
|
Abstract
Objective
The present investigation was aimed to explore the potential of lactobacilli for conjugated linoleic acid (CLA) production, isolated from rumen fluid samples of lactating goats.
Methods
A total of 64 isolates of lactobacilli were obtained using deMan-Rogosa-Sharpe (MRS) agar from rumen fluid of goats and further subjected to morphological and biochemical characterizations. Isolates found as gram-positive, catalase negative rods were presumptively identified as Lactobacillus species and further confirmed by genus specific polymerase chain reaction (PCR). The phylogenetic tree was constructed from the nucleotide sequences using MEGA6.
Results
Out of the 64 isolates, 23 isolates were observed positive for CLA production by linoleate isomerase gene-based amplification and quantitatively by UV-spectrophotometric assay for the conversion of linoleic acid to CLA as well as gas chromatography-based assay. In all Lactobacillus species cis9, trans11 isomer was observed as the most predominant CLA isomer. These positive isolates were identified by 16S rRNA gene-based PCR sequencing and identified to be different species of L. ingluviei (2), L.salivarius (2), L. curvatus (15), and L. sakei (4).
Animal food and food products of ruminant origin are the major sources of nutrients, however, in some cases they are considered to be harmful to human health due to high risk for cardiovascular diseases as they have high level of saturated fatty acids (FA) and cholesterol [1]. Furthermore, ruminant fat both in milk and meat contains many specific FA such as vaccenic acid, n-3, n-6 poly unsaturated fatty acids and conjugated linoleic acid (CLA), which are associated with their various health promoting properties. The CLA is a mixture of 28 positional and geometric isomers of linoleic acid (LA) present in animal products having various nutraceutical characteristics [2–4]. It is formed mainly as an intermediate during the biohydrogenation of LA in the rumen [5] or from the endogenous conversion of trans-vaccenic acid in the mammary gland [6]. The potent biological effect of CLA such as anti-carcinogenic, anti-inflammatory, anti-obesity, anti-diabetic, immunomodulatory, anti-atherosclerotic, reduction of whole-body fat and bone formation promoting properties have been identified in a wide range of animal species [2,4,7]. The recommended dietary allowance of CLA for humans ranges from 1 to 3 g/d to accomplish desired health benefits [8]. However, the availability (36 to 440 mg/d) is very much lower than the requirement to exert the beneficial effects. Chemical synthesis by alkaline isomerization of LA rich oil to produce CLA is quite expensive and yields unenviable side products. Although ruminant derived products are the richest source of CLA in the human diet, still the concentration present in the foodstuffs is too low and depends upon feedstock and type of animal breed [9]. Therefore, there is an ever-increasing interest to augment this constituent in animal food products for better health aspects.
Dietary intervention and strategies for animal management have the possibility of creating rumen environment that alters FAs biohydrogenation and enhance CLA content in milk. Rumen microbial fermentation is found to improve the CLA concentration in animal products [10]. Hence, there is an opportunity to further increase the CLA content of animal products by employing these rumen microbes [11]. This approach can be feasible by adding the ruminant’s diet rich in LA and/or by supplementing the microbes directly into the rumen having potential of CLA production which ultimately lead to foods of animal origin with enhanced nutraceutical importance. A number of CLA producing bacteria of the rumen origin including Butyrivibrio fibrisolvens [12], lactic acid bacteria (LAB) namely Lactobacillus acidophilus, Lactococcus lactis subsp. cremoris, L. lactis subsp. lactis, L. delbrueckii subsp. bulgaricus, L. delbrueckii subsp. Lactis and have been reported to convert LA into CLA [11]. However, information is lacking regarding CLA production from Lactobacillus species of rumen origin of Indian breeds except few reports despite the well-known fact that LAB possess generally recognized as safe status and can be applied as probiotics in animal health improvement. The present study, therefore, was conducted to isolate, identify and characterize the CLA producing lactobacilli from rumen samples of AXB goats.
The rumen sampling was performed from crossbreed lactating goats (Anglo Nubian×Beetal; age ~3 years; weight ~30 kg) maintained at Livestock Research Centre, ICAR-National Dairy Research Institute, Karnal-132001, Haryana. The goats were not fed with any probiotics. Around 100 mL of rumen fluid was collected using a suction pump and immediately transported to the laboratory. Samples were collected in O2 free CO2 flushed sterilized containers and homogenized before further processing. One mL of each sample was suspended in deMan-Rogosa-Sharpe (MRS) broth, containing LA (0.5 mg/mL) and incubated at 39°C for 24 h [13]. Samples were serially diluted in peptone water (0.1%) and subsequently plated on MRS agar with incubation up to 3 days at 39°C. Colonies were picked up randomly to MRS broth and the isolates were streaked on MRS agar plates for further purification. The purity of cultures was examined microscopically after performing Gram-staining and preserved at −80°C in glycerol stocks (10%). For routine use, the isolates were maintained in MRS broth at 4°C and freshly activated at each time before use.
Molecular confirmation of the isolates as lactobacilli was done after DNA isolation followed by polymerase chain reaction (PCR) amplification using genus specific primers. Genomic DNA was isolated as per the modified methods of Jena et al [14] from MRS broth of respective isolates. Briefly, the bacterial cell suspension was transferred to a 2 mL microcentrifuge tube and pelleted by centrifugation at 8,000×g for 10 min. Then 800 μL cetyl trimethyl ammonium bromide extraction buffer was added to the cells and mixed thoroughly. The cell suspension was incubated at 70°C for 1 h and vortexed every 15 min of interval. A volume of 500 μL of chloroform:isoamyl alcohol (24:1) was added to the suspended pellet and mixed upside down to form a white emulsion. In the next step, centrifugation was carried out at 8,000×g for 20 min. The aqueous layer was transferred, and DNA was precipitated with addition of 300 μL of isopropanol and centrifuged (8,000×g, 10 min). Subsequently, 500 μL of 70% ethanol was added to the white pellet with mixing. After centrifugation pellet was air dried and suspended in Tris-ethylenediaminetetraacetic acid (Tris-EDTA) buffer (pH 8.0) and incubated at 60°C for 10 min. The quality of isolated DNA was checked by 0.8% agarose gel electrophoresis and concentration was quantified by Nano drop plate reader (Tecan-Infinite Pro 200, Männedorf, Switzerland) and thereafter, stored at −20°C for further use.
For identification of bacterial isolates at genus level, PCR was performed with the following primer pairs and PCR conditions [15]. The PCR reaction mixture (50 μL) contained dNTPs each 2.5 mM; each primer 20 pmol; 10× PCR bufer 5 μL; Taq DNA polymerase (Genetix, New Delhi, India) 1 U and template DNA 100 ng. The forward primer was LbLMA1-rev (5′-CTCAAAACTAAACAAAGTTTC-3′) and the reverse primer was R16-1(5′-CTTGTACACACCGCCCGTCA-3′). PCR amplification was carried out in a thermal cycler (Quanta Biotech-96, Beverly, MA, USA) with initial denaturation of 94°C for 5 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 45 s and extension at 72°C for 30 s, followed by a final extension at 72°C for 7 min. Amplified DNA fragments were examined by horizontal agarose gel electrophoresis containing ethidium bromide (0.5 μg/mL) at 100 V for 1 h in 1× Tris-Borate-EDTA bufer with 5 μL aliquots of PCR products. The gel images were digitized through Genview (New Delhi, India), Genetix (India), Biotech Asia Pvt. Ltd. (New Delhi, India). Further, all the positive isolates were checked for PCR based linoleate isomerase (LAI) gene amplification.
The presence of the LAI gene responsible for the conversion of LA to CLA was checked by PCR based amplification as per the earlier methods [16]. Briefly, PCR amplification of target gene was carried using the following set of primers LISO3 - (5′-CGGACNTACGTYGAYTTAATGG-3′); and LISO4- (5′-TGGTGMACMACRATCGACAT-3′) containing a reaction volume of 25 μL (2.5 mM dNTPs each; each primer 10 pmol; 10× PCR bufer 2.5 μL; Taq DNA polymerase [Genetix, India] 0.5 U; 1 μL MgCl2 [20 mM] and template DNA 50 ng). PCR amplification was carried out in a thermal cycler (Quanta Biotech-96, USA) with initial denaturation of 94°C for 5 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 45 s and extension at 72°C for 1 min, followed by a final extension at 72°C for 5 min. Amplified DNA fragments were examined by horizontal agarose gel electrophoresis and gel images were digitized as described in previous section. All the positive isolates were further screened for CLA production assay from LA by spectrophotometric methods.
After PCR confirmation lactobacilli isolates were further characterized for CLA biosynthesis in MRS broth using the UV-based spectrophotometric assay. A stock solution of LA (5 mg/mL, 99% purity; Sigma-Aldrich, St. Louis, MO, USA) was prepared in sterile distilled water with 1% (w/v) Tween-80 (Hi-media, Mumbai, India) and sterilised through a 0.2 μm syringe filter. For screening, the cultures were inoculated at 1% (v/v) to 10 mL MRS broth supplemented with 0.05% L-cys-HCl and 0.5 mg/mL of LA as a substrate and incubated at 37°C for 24 h. Subsequently, the lactobacilli isolates were tested for the efficacy to produce CLA in accordance to Barrett et al [17]. Briefly, the samples were centrifuged at 13,000 rpm at 4°C for 5 min, the supernatant (1 mL) was vigorously mixed with 2 mL of isopropanol and left undisturbed for 3 min. To this, 1.5 mL of hexane was added for extraction of FAs and remained undisturbed for 3 min. An aliquot 1 mL was taken for absorbance at 233 nm in UV-VIS spectrophotometer (Specord-200, Schönwalde-Glien, Germany). A standard curve (0.5 to 10 μg/mL) was prepared from reference trans10, cis12 CLA isomer to quantify total CLA and hexane layers containing only LA were used as blank in the entire assay.
Fatty acid methyl esters (FAME) was prepared following direct synthesis method of O’Fallon et al [18] with little modifications. The FA analysis of MRS medium was performed directly on the liquid solutions. Lactobacilli-MRS medium was hydrolyzed for 1.5 h at 55°C in 10 N potassium hydroxide (KOH) in methanol. C19:0 in methanol was used as an internal standard. The KOH is neutralized, and the free FAs were methylated by sulfuric acid catalysis for 1.5 h at 55°C. Hexane was then added to the reaction tube, which was vortex-mixed and centrifuged. The hexane layers containing FAs were dried under a stream of N2 and re-dissolved in minimum volume of hexane. The samples were stored in glass vials at −20°C until analysed in gas chromatography (GC).
One μL sample (FAME) was injected into a fully automated Bruker 450 GC machine (Bruker Daltonik GmbH, Bremen, Germany) equipped with Rtx-2330 capillary column (120 m×0.25 mm I.D., 0.20 μm film thickness, Restek corporation, Bellefonte, PA, USA), an automated injector and a flame ionization detector in (1:50) split mode using hydrogen as a carrier gas. The temperatures of injector and detector were set at 260°C and 270°C, respectively. The temperature of column oven was programmed from 170°C to 240°C with step increase of 4°C/min. The quantitative analysis of CLA isomers was performed by comparison of retention times with methylated CLA standards (cis9, trans11; trans10, cis12 and trans9, trans11). The FAMEs were identified by comparing retention times with those of known standards. The areas of the individual peaks were used to determine the relative percentage of each FA present in the samples.
The DNA samples of the positive isolates confirmed for CLA production were further amplified with universal 16S rRNA gene primers; 27F 5′-AGAGTTTGATCCTGGCTCAG and 1492R 5′-GGTTACCTTGTTACGACTT [19]. The universal primers targeting partial 16S rRNA gene region were used to amplify the PCR reaction. The PCR components (10× PCR buffer; 10 μM each primer; 10 mM each dNTPs mix; Taq DNA polymerase 1 U; template DNA 100 ng) were mixed properly and the reaction was executed in a 50 μL of PCR reaction mixture under normal PCR cycling conditions for 35 cycles. The amplified PCR products were gel purified with QIA quick Gel extraction kit (Cat#28704) as per the manufacturer’s instructions (QIAGEN, Hilden, Germany) and out sourced for sequencing in both directions (Eurofins Genomics, India, Pvt. Ltd., Bengaluru, Karnataka, India). The sequences received from the chromatogram were retrieved and verified in BioEdit software and both strands were aligned with CLUSTAL W. Then the sequences were BLAST analysed to search for the sequence resemblances with other Lactobacillus sequences (http://blast.ncbi.nlm.nih.gov/) and were submitted to NCBI GenBank using BankIt submission tool. All the cultures were provided with accession numbers were recorded (MF148304-07 and MG430183-201). The phylogenetic tree was constructed from the nucleotide sequences using MEGA6 and 7 references sequences (MF992225, GU125609, LC 130553, KC416998, MG266175, FJ378897, AB911500, and MG430201) were included in this analysis.
Out of all the positive isolates, 19 isolates failed to differentiate on the basis of 16S rRNA gene sequence analysis, so further for species level confirmation biochemical tests viz. xylose and mellibiose fermentation and arginine hydrolysis was executed in basal medium (MRS medium without carbohydrate source) supplemented with 1% of each substrate.
A total of 64 isolates were isolated on selective media (MRS Agar) supplemented with LA from rumen fluid content of goats. Rice straw shaped colonies were observed morphologically on MRS agar plates and microscopically isolates were found to be Gram positive rods with negative catalase and oxidase activity, a common characteristic of Lactobacillus (Table 1). These isolates were designated as goat isolates (GI) followed by isolate number (Figure 1). Molecular level confirmation based on genus specific PCR amplification showed a product size of approximately 220 bp with all the isolates. The similar set of primers (LbLMA1 and rev/R16-1) was also used by many researchers to confirm Lactobacillus spp. coding for a spacer region between the 16S and 23S rRNA genes [13]. These positive isolates confirmed at genus level were further observed for LAI gene-based PCR amplification. In a number of studies, it was reported LAI gene to be a multi-component enzyme system, encoded in the genome of lactobacilli responsible for the biohydrogenation of LA to CLA with various intermediate steps [2,16,20]. Out of 64 isolates only 23 isolates showed a positive PCR amplification with desired product size around 968 bp.
All the 23 positive isolates were further studied quantitatively for the production of CLA in MRS broth supplemented with LA with active cultures of these isolates by UV-spectrophotometric based assay [17]. After 24 h of conversion, all the isolates were observed to produce 26.03 to 43.62 μg/mL of CLA from LA (Figure 2). Highest production of CLA was observed with GI-52 while lowest production was recorded from the isolate GI-23. Only 3 isolates (GI-19, GI-46, and GI-52) showed more than 40 μg/mL of CLA and most of the isolates showed an average 30 μg/mL of CLA in MRS supplemented broth.
Although the accurate pathway of CLA synthesis is still obscure, nevertheless, it has been observed that a LA detoxifying mechanism is responsible to eradicate the toxic effects of the substrate for cell survival as the assimilation of LA into bacterial cell membrane changes the membrane potential, lipid bilayer chemistry and intra-membrane machinery [9]. Highest CLA production (95.25 μg/mL) has been observed in Lactobacillus plantarum (L. plantarum) amongst lactobacilli strains by Khosravi et al [21]. Kishino et al [22] reported the potential of lactobacilli strains with high CLA production ranging from 3.41 mg/mL to 0.07 mg/mL. Our results and variation of CLA are in concurrence with observations of earlier studies of LAB isolates [23]. Six isolates of L. plantarum with minor variations in CLA conversions from 3.85% to 4.90% in traditional dairy origin was also reported [24]. The results strongly support with the fact that strain variation in lactobacilli has different ability to release CLA from LA. In our study, though higher production of CLA was observed, the source of isolation and strain variation may have higher degree of CLA producing capabilities.
Health beneficial effects of CLA are specific to their respective isomers, hence proper analysis is necessary prior to use. All the 23 lactobacilli isolates having CLA production were studied for specific isomer production in MRS medium supplemented with desired LA. Out of all the lactobacilli strains 12 isolates were observed to produce c9,t11; t10,c12; and t9,t11 CLA isomers in the mediums. In all 23 lactobacilli c9,t11 isomer was observed as the most predominant isomer. The observation of the current study is in agreement with previous findings of isomer variability [9,11,23]. Strains GI-15, GI-35, GI-40, GI-44, and GI-54 did not show the production of t9, t11 isomers, whereas, few strains (GI-45, GI-49, and GI-56) showed the presence of c9,t11 and t9,t11 isomers and no t10, c12 isomer production. The findings are also substantiated by earlier observations that biosynthesis of t9,t11 CLA isomer was a biotransformation consequence of c9,t11 CLA [13].
The observations of this study indicated that synthesis isomers of CLA are strain dependent. Lee et al [25] reported production of c9, t11 (26.8 μg/mL) and t10, c12 (6.4 μg/mL) isomers from L. plantarum PL62 isolated from infant faces. Eight different CLA isomers with the enzyme extract of L. acidophilus CCRC 14079 with LA (t8,t10; t9,t11; t10,t12; t11,t13; t8,c10; c9,t11; t10,c12; and c11,t13) have been reported by Lin et al [26]. In connection to these findings, Sosa-Castañeda et al [9] assessed the CLA production potential of 13 strains of lactobacilli isolates and reported L. fermentum J20 to produce c9, t11 (42.63 μg/mL) and t10,c12 (8.27 μg/mL) CLA isomers. In another study, L. plantarum JCM 1551 was reported to produce 2.4 μg/mL of CLA, comprised of c9,t11 (21%) and t9, t11 (79%) CLA isomers [27] which are in contrast to our observations. The biotransformation of LA to CLA isomers is an isomerization process of linoleate isomerase enzyme [20] that is a multi-component enzymatic system responsible for biohydrogenation activity. The variability of isomers observed in CLA production supported varying capability of strains of different isomeric forms of linoleate isomerase enzyme [28]. In overall, isolate GI-52 was observed to be the most competent producer of CLA in terms total CLA production.
All the positive isolates were 16S rRNAgene amplified and showed a product size of approximately 1,500 bp. BLAST analysis revealed more than 99% homology with existing sequences. Phylogenetic tree constructed from all the sequences differentiated the isolates into 3 different clusters (Figure 1). Out of 23 isolates Cluster-I comprises of 19 isolates, whereas Cluster-II and III comprises 2 isolates in each group respectively. In Cluster-I all the isolates grouped together with reference sequences of Lactobacillus curvatus (L. curvatus) and Lactobacillus sakei (L. sakei) and could not differentiate into separate groups with a similarity of more than 99% homology. These results are in agreement with many findings, where it was reported that it is hard to discriminate L. curvatus and L. sakei as the genetic heterogeneity within the species is nearly similar [29,30]. Based on phenotypic and genotypic properties [31], primers developed from random amplified polymorphic DNA fingerprints and multiplex PCR-based analysis [30], were used to differentiate in between these species. Hence, based on melibiose fermentation and ammonia liberation from arginine hydrolysis, the isolates were differentiated into L. curvatus and L. sakei (Table 1). In Cluster-II, 2 of our isolates were grouped with Lactobacillus salivarius (L. salivarius), whereas Cluster-III isolates (2) showed similarity with Lactobacillus ingluviei (L. ingluviei) (Figure 1).
Earlier reports have showed the production of CLA by LAB [21]. Lower production of CLA was reported by strains of L. curvatus and L. sakei (4.2% and 1.6%, respectively), as meat fermentation starter cultures or as natural microorganism [32]. In the same study it was also observed that conjugated linolenic acid (CLNA) produced by other LAB strains of L. curvatus and L. sakei with 60.1% and 22.4% conversion respectively [32]. For L. curvatus and L. sakei strains, a poor conversion percentage of LA into CLA was reported in MRS medium in contrast to high conversion of LNA into CLNA [32]. The presence of CLA producing L. salivarius was observed in a diverse group of samples. L. salivarius have been isolated from animal origin [33] and applied as the direct fed microbial starter for the early development of calf rumen [34]. Ting et al [35] characterized CLA producing LAB as the potential probiotic for chicken. Dahiya and Puniya [13] reported L. salivarius isolated from dairy based products and infant fecal origin and showed to produce CLA less than 25 μg/mL. Isolate CCB1 (L. salivarius strain P2) isolated from chicken was observed to produce 21.97 μg/mL of CLA after 48 h conversion [35]. Although the production of CLA in our study is little higher, it is defensible as the production is highly strain specific [26]. The isolates of L. ingluviei are a group of LAB studied in the gastrointestinal tracts of birds [36] however their presence in goat rumen fluid it is limited to our study only. The CLA production and its effects are so far not reported from any other sources to the best of our knowledge.
The study showed that lactobacilli isolated from goat rumen fluid have the potential to produces bioactive isomers of CLA. These isolates can be applied as direct fed microbial as they have generally recognized as safe status and easier to handle than other known CLA producers in the rumen. The isolates can be further studied for their probiotic potential and may be applied as ruminant feed additives to enhance the nutraceutical value of milk. However, more studies are required to validate these finding in suitable animal trials and their isomer specific health benefits.
ACKNOWLEDGMENTS
The authors are thankful to Director, ICAR-NDRI, Karnal for necessary facilities to conduct the research work. The financial assistance provided by DBT (BT/PR/15038/TRM/120/59/2015) to carry out this research work is gratefully acknowledged.
Figure 1
Evolutionary relationships of isolated conjugated linoleic acid producing Lactobacillus species isolated from rumen samples of goat by phylogenetic tree constructed from all the sequences.

Figure 2
Comparative conjugated linoleic acid production (in μg/mL) assay of the positive Lactobacillus isolates after 24 h incubation.
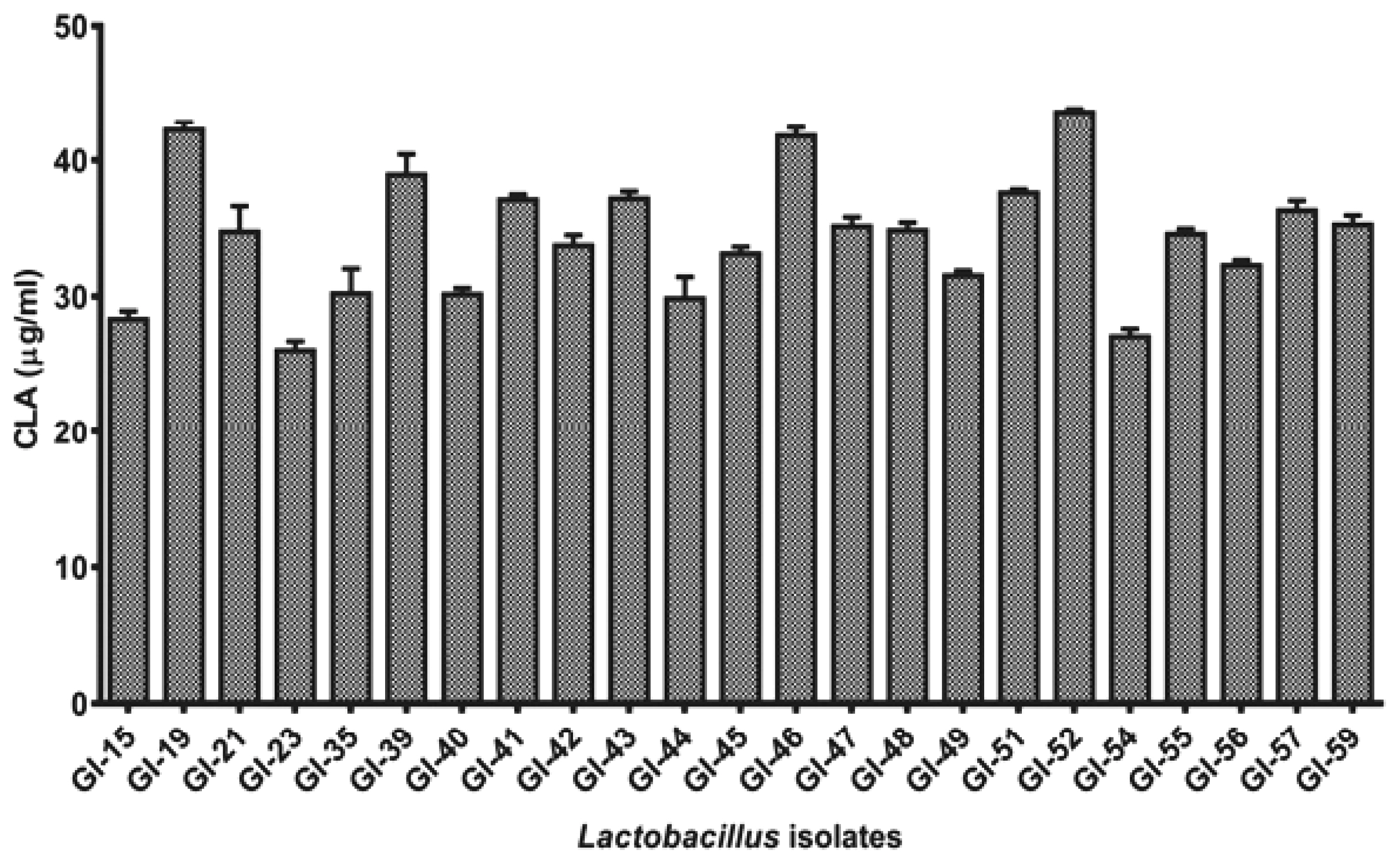
Table 1
Biochemical characteristics and identification of the conjugated linoleic acid positive isolates
REFERENCES
1. German JB, Gibson RA, Krauss RM, et al. A reappraisal of the impact of dairy foods and milk fat on cardiovascular disease risk. Eur J Nutr 2009; 48:191–203.
https://doi.org/10.1007/s00394-009-0002-5



2. Kim L, Park Y, Park Y.
trans-10,cis-12 CLA promotes osteoblastogenesis via SMAD mediated mechanism in bone marrow mesenchymal stem cells. J Funct Foods 2014; 8:367–76.
https://doi.org/10.1016/j.jff.2014.04.006



3. Yang B, Chen H, Gu Z, et al. Synthesis of conjugated linoleic acid by the linoleate isomerase complex in food-derived lactobacilli. J Appl Microbiol 2014; 117:430–9.
https://doi.org/10.1111/jam.12524



4. Yang B, Chen H, Stanton C, et al. Review of the roles of conjugated linoleic acid in health and disease. J Funct Food 2015; 15:314–25.
https://doi.org/10.1016/j.jff.2015.03.050

5. Kepler CR, Hirons KP, McNeill JJ, Tove SB. Intermediates and products of the biohydrogenation of linoleic acid by Butyrinvibrio Fibrisolvens
. J Biol Chem 1966; 241:1350–4.


6. Corl BA, Baumgard LH, Dwyer DA, Griinari JM, Phillips BS, Bauman DE. The role of Δ9-desaturase in the production of cis-9, trans-11 CLA. J Nutr Biochem 2001; 12:622–30.
https://doi.org/10.1016/S0955-2863(01)00180-2


7. Benjamin S, Prakasan P, Sreedharan S, Wright AD, Spener F. Pros and cons of CLA consumption: an insight from clinical evidences. Nutr Metab 2015; 12:4
https://doi.org/10.1186/1743-7075-12-4

8. Macdonald HB. Conjugated linoleic acid and disease prevention: a review of current knowledge. J Am Coll Nutr 2000; 19:111S–8S.
https://doi.org/10.1080/07315724.2000.10718082


9. Sosa-Castañeda J, Hernández-Mendoza A, Astiazarán-García H, et al. Screening of Lactobacillus strains for their ability to produce conjugated linoleic acid in milk and to adhere to the intestinal tract. J Dairy Sci 2015; 98:6651–9.
https://doi.org/10.3168/jds.2014-8515


10. Griinari JM, Bauman DE. Biosynthesis of conjugated linoleic acid and its incorporation into meat and milk in ruminants. Yurawecz MP, Mossoba MM, Kramer JKG, Pariza MW, Nelson GJ, editorsAdvances in conjugated linoleic acid research. Champaign, IL, USA: AOCS Press; 1999. p. 180–201.
11. Puniya AK, Chaitanya S, Tyagi AK, De S, Singh K. Conjugated linoleic acid producing potential of lactobacilli isolated from the rumen of cattle. J Ind Microbiol Biotechnol 2008; 35:1223–8.
https://doi.org/10.1007/s10295-008-0429-3



12. Asraf Hussain SK, Srivastava A, Tyagi A, et al. Characterization of CLA-producing Butyrivibrio spp. reveals strain-specific variations. 3 Biotech 2016; 6:90
https://doi.org/10.1007/s13205-016-0401-2



13. Dahiya DK, Puniya AK. Isolation, molecular characterization and screening of indigenous lactobacilli for their abilities to produce bioactive conjugated linoleic acid (CLA). J Food Sci Technol 2017; 54:792–801.
https://doi.org/10.1007/s13197-017-2523-x




14. Jena R, Choudhury PK, Puniya AK, Tomar SK. Isolation and species delineation of genus Bifidobacterium using PCR-RFLP of partial hsp60 gene fragment. LWT-Food Sci Technol 2017; 80:286–93.
https://doi.org/10.1016/j.lwt.2017.02.032

15. Dubernet S, Desmasures N, Guéguen M. A PCR-based method for identification of lactobacilli at the genus level. FEMS Microbiol Lett 2002; 214:271–5.
https://doi.org/10.1111/j.1574-6968.2002.tb11358.x



16. Gorissen L, Weckx S, Vlaeminck B, et al. Linoleate isomerase activity occurs in lactic acid bacteria strains and is affected by pH and temperature. J Appl Microbiol 2011; 111:593–606.
https://doi.org/10.1111/j.1365-2672.2011.05087.x


17. Barrett E, Ross R, Fitzgerald G, Stanton C. Rapid screening method for analyzing the conjugated linoleic acid production capabilities of bacterial cultures. Appl Environ Microbiol 2007; 73:2333–7.
http://doi.org/10.1128/AEM.01855-06



18. O’Fallon JV, Busboom JR, Nelson ML, Gaskins CT. A direct method for fatty acid methyl ester synthesis: application to wet meat tissues, oils, and feedstuffs. J Anim Sci 2007; 85:1511–21.
https://doi.org/10.2527/jas.2006-491



19. Lane DJ. 16S/23S rRNA sequencing. Stackebrandt E, Goodfellow M, editorsNucleic acid techniques inbacterial systematics. New York, NY, USA: John Wiley; 1991. p. 115–76.
20. Ogawa J, Matsumura K, Kishino S, Omura Y, Shimizu S. Conjugated linoleic acid accumulation via 10-hydroxy-12-octadecaenoic acid during microaerobic transformation of linoleic acid by Lactobacillus acidophilus
. Appl Environ Microbiol 2001; 67:1246–52.
https://doi.org/10.1128/AEM.67.3.1246-1252.2001



21. Khosravi A, Safari M, Khodaiyan F, Gharibzahedi SMT. Bioconversion enhancement of conjugated linoleic acid by Lactobacillus plantarum using the culture media manipulation and numerical optimization. J Food Sci Technol 2015; 52:5781–9.
https://doi.org/10.1007/s13197-014-1699-6




22. Kishino S, Ogawa J, Omura Y, Matsumura K, Shimizu S. Conjugated linoleic acid production from linoleic acid by lactic acid bacteria. J Am Oil Chem Soc 2002; 79:159–63.
https://doi.org/10.1007/s11746-002-0451-4

23. Andrade JC, Ascencao K, Gullon P, et al. Production of conjugated linoleic acid by food-grade bacteria: a review. Int J Dairy Technol 2012; 65:467–81.
https://doi.org/10.1111/j.1471-0307.2012.00871.x

24. Li H, Liu Y, Liu X, Zhang H. Conjugated linoleic acid conversion by six Lactobacillus plantarum strains cultured in MRS broth supplemented with sunflower oil and soymilk. J Food Sci 2012; 77:M330–6.
https://doi.org/10.1111/j.1750-3841.2012.02723.x


25. Lee K, Paek K, Lee H, Park JH, Lee Y. Antiobesity effect of trans-10, cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J Appl Microbiol 2007; 103:1140–6.
https://doi.org/10.1111/j.1365-2672.2007.03336.x


26. Lin TY, Lin CW, Wang YJ. Production of conjugated linoleic acid by enzyme extract of Lactobacillus acidophilus CCRC 14079. Food Chem 2003; 83:27–31.
https://doi.org/10.1016/S0308-8146(03)00032-3

27. Ando A, Ogawa J, Kishino S, Shimizu S. CLA production from ricinoleic acid by lactic acid bacteria. J Am Oil Chem Soc 2003; 80:889–94.
https://doi.org/10.1007/s11746-003-0790-1

28. Farmani J, Safari M, Roohvand F, Razavi SH, Aghasadeghi MR, Noorbazargan H. Conjugated linoleic acid-producing enzymes: a bioinformatics study. Eur J Lipid Sci Technol 2010; 112:1088–100.
https://doi.org/10.1002/ejlt.201000360

29. Koort J, Vandamme P, Schillinger U, Holzapfel W, Björkroth J.
Lactobacillus curvatus subsp. melibiosus is a later synonym of Lactobacillus sakei subsp. carnosus
. Int J Syst Evol Microbiol 2004; 54:1621–6.
https://doi.org/10.1099/ijs.0.63164-0


30. Lee J, Jang J, Kim B, Kim J, Jeong G, Han H. Identification of Lactobacillus sakei and Lactobacillus curvatus by multiplex PCR-based restriction enzyme analysis. J Microbiol Methods 2004; 59:1–6.
https://doi.org/10.1016/j.mimet.2004.05.004


31. Klein G, Dicks LMT, Pack A, et al. Emended descriptions of Lactobacillus sake (Katagiri, Kitahara, and Fukami) and Lactobacillus curvatus (Abo-Elnaga and Kandler): numerical classification revealed by protein fingerprinting and identification based on biochemical patterns and DNA-DNA hybridizations. Int J Syst Bacteriol 1996; 46:367–76.
https://doi.org/10.1099/00207713-46-2-367

32. Gorissen L, Leroy F, De Vuyst L, De Smet S, Raes K. Bacterial production of conjugated linoleic and linolenic acid in foods: a technological challenge. Crit Rev Food Sci Nutr 2015; 55:1561–74.
https://doi.org/10.1080/10408398.2012.706243


33. Seo JK, Kim SW, Kim MH, Upadhaya SD, Kam DK, Ha JK. Direct-fed microbials for ruminant animals. Asian-Australas J Anim Sci 2010; 23:1657–67.
https://doi.org/10.5713/ajas.2010.r.08


34. Frizzo LS, Sotto LP, Zbrun MV, et al. Lactic acid bacteria to improve growth performance in young calves fed milk replacer and spray-dried whey powder. Anim Feed Sci Technol 2010; 157:159–67.
https://doi.org/10.1016/j.anifeedsci.2010.03.005

35. Ting YS, Saad WZ, Chin SC, Wan HY. Characterization of conjugated linoleic acid-producing lactic acid bacteria as potential probiotic for chicken. Malays J Microbiol 2016; 12:15–23.
https://dx.doi.org/10.21161/mjm.67214






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





