 |
 |
| Anim Biosci > Volume 31(6); 2018 > Article |
|
Abstract
Objective
The objective of this experiment was to compare the effects of adding 130 mg/kg Cu from either copper sulfate (CS) or tribasic copper chloride (TBCC) on growth performance, mineral deposition in tissues, and the excretion in feces of pigs as well as changes in the mineral contents in tissues and feces when the supplemental Cu level was decreased from 130 mg/kg to 10 mg/kg.
Methods
A total of 72 pigs (32.6┬▒1.2 kg) were randomly assigned to a CS diet or a TBCC diet with 6 pens per treatment. The trial lasted 102 d and included 3 phases (phase 1, 1 to 30 d; phase 2, 31 to 81 d; and phase 3, 82 to 102 d). The supplemental levels of Cu in the 2 treatments were 130 mg/kg in phase 1 and 2 and 10 mg/kg in phase 3.
Results
The results showed that pigs fed the CS diet tended to have higher average daily gain than pigs fed the TBCC diet during d 1 to 81 (p<0.10). Compared with CS, TBCC increased the activities of aspartate transaminase (AST), ceruloplasmin, and superoxide dismutase in serum on d 30 (p<0.05). The TBCC decreased the Cu level in the liver on d 81 (p<0.05) and increased the Mn level in the liver on d 102 (p<0.05). The concentration of Cu in feces sharply decreased when the supplemental Cu level in diet changed from 130 mg/kg to 10 mg/kg in both diets (p<0.05).
Copper is an essential element for swine and plays important roles in many physiological metabolic processes. Dietary Cu levels of 5 to 10 mg/kg are generally enough to meet the pigŌĆÖs nutrient requirement for these processes according to the NRC [1]. High dietary levels of Cu (100 to 250 mg/kg) have proven to have positive effects on improving growth performance in weanling piglets and were applied to swine production [2,3]. However, long-term supplementation of a high level of Cu can create a high risk of Cu accumulation in organs as well as ecological environment, which needs more attention. The maximum permitted level of Cu in food is 10 mg/kg according to the hygienic standard for meat products of Chinese Regulation [4]. High levels of Cu accumulation in organs will harm humanŌĆÖs health because most people in China have the habit of using the organs of pigs as food, especially liver and kidney. Furthermore, a high level of Cu would influence the metabolism of other minerals by the interaction of Cu with other mineral elements.
The most common form of Cu used in feeds for growth promotion in pigs is copper sulfate (CS). Tribasic copper chloride (TBCC) is a less reactive and less destructive form of Cu due to its low hygroscopicity when combined with vitamins in base mixes, supplements, and diets. In vitro digestion assay showed that the solubility of Cu was not different between the sources [5]. Previous research indicated that TBCC could improve growth performance during early and late finishing [2] but that CS had no growth promoting benefit during finishing periods [6]. At the same time, data is limited about the effects of CS and TBCC on minerals deposition in tissues and excretion in feces. Therefore, the objective of this experiment was to compare the effects of adding 130 mg/kg Cu from either CS or TBCC on growth performance, trace mineral deposition in tissues, and the excretion in feces of pigs as well as changes in the mineral contents in tissues and feces when the supplemental Cu level was decreased from 130 mg/kg to 10 mg/kg.
The experimental protocol used in the following experiment was reviewed and approved by the Animal Experimental Committee of Sichuan Agricultural University. This experiment was conducted at the Animal Experiment Center of Chengdu Shuxing Feed Co. Ltd. Copper sulfate and TBCC were provided by Chengdu Shuxing Feed Co. Ltd. (Chengdu, China).
A total of 72 crossbred Duroc├ŚLandrace├ŚYorkshire pigs (70┬▒1 d and 32.6┬▒1.2 kg) were assigned to the CS diet or the TBCC diet with 6 replicate pens per treatment and six pigs per pen (3 barrows and 3 gilts per pen) in a randomized complete block design according to initial body weight (BW) and gender. The trial lasted 102 d and included 3 phases (phase 1, 30 to 50 kg [1 to 30 d]; phase 2, 50 to 100 kg [31 to 81 d]; and phase 3, 100 to 110 kg [82 to 102 d]). The concentrations of supplemental Cu in the 2 treatments were 130 mg/kg in phase 1 and 2 and 10 mg/kg in phase 3. The basal diets were formulated to meet or exceed NRC nutrient recommendations [1]. Ingredient compositions of the basal diets are presented in Table 1. Before the experiment, all pigs were fed corn-soybean diet supplementation with 6 mg/kg CS. All pigs were housed in a temperature-controlled room with continuous lighting. Each pen (4 by 3 m) was equipped with a 1-sided feeder and a stainless-steel nipple drinker to allow the pig ad libitum access to feed and water. All pigs were individually weighed at the beginning of experiment and the end of each phase after 12 h of fasting, and feed intake per pen was recorded daily throughout the experiment to calculate average daily gain (ADG), average daily feed intake, and gain/feed.
Representative feed samples of each dietary treatment were collected for chemical analysis. Approximately 200 g of feed samples from each diet were dried at 65┬░C for 48 h and subsequently ground to pass through a 1-mm screen. All feed samples were analyzed for CP, Ca, and P according to official methods of analysis of AOAC [7].
At the end of phase 1 and phase 2 of the experiment, blood samples were collected into glass tubes without anticoagulant by jugular venipuncture from one selected male pig with BW closet to average BW in each pen. After centrifugation (3,000├Śg for 15 min at 4┬░C), serum samples were collected and stored at ŌłÆ20┬░C until analysis.
On d 0, 3, 7, 14, and 21 of phase 3, fresh fecal samples were randomly collected from at least 2 pigs in each pen twice in a day. The fresh fecal samples were marked with pen number and date and then stored at ŌłÆ20┬░C before drying. Approximately 200-g fecal samples from each pen were dried at 65┬░C for 48 h and subsequently ground to pass through a 1-mm screen and stored in plastic bags at ŌłÆ20┬░C until analysis.
At the end of phase 2 and phase 3, one male pig with BW close to average BW in each pen was chosen to sample. Pigs were anesthetized by electric shock and then euthanized by exsanguination. The abdomen was immediately opened for the collection of liver, kidney, and longissimus dorsi muscle samples. Before analysis, samples were stored at ŌłÆ20┬░C.
Serum total protein, albumin, urea, aspartate transaminase (AST), and alanine transaminase (ALT) were measured by the UV method using an automatic biochemistry analyzer (Hitachi 7020; Hitachi, Ltd., Chiyoda, Tokyo, Japan).
The activities of glutathione peroxidase (GPx), superoxide dismutase (SOD) and ceruloplasmin in serum were measured by assay kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The methods were according to the manufacturerŌĆÖs instructions. All samples were measured in duplicate.
The contents of Cu in serum and Cu, Fe, Mn, and Zn in liver, kidney, muscle, and feces were measured by atomic absorption spectrometry (GGX-6, Bejing Kechuang Haiguang instrument Co. Ltd., Beijing, China). The tissue and feces samples were prepared for mineral elements analysis by wet ashing using nitric acid and perchloric acid [8]. Before analysis, samples were dried overnight at 105┬░C. Approximately 2.0 g of tissues and feces were obtained from each sample for this purpose. The concentrations of trace minerals in tissues and feces are expressed as milligrams per kilogram of dry matter (DM).
Replicate pen was considered the experimental unit for all analysis (n = 6). Data were analyzed by using independent sample t test comparisons and by 1-way analysis of variance for the different time points of mineral contents in feces within each type of diet. The results were presented as means and standard error of the mean. For significance determination, the ╬▒-level was set as 0.05. Possibility value Ōēż0.05 indicated significant difference, and values between 0.05 and 0.1 indicate a trend. All statistical analysis was performed using SPSS 19.0 (IBM, Armonk, NY, USA).
The effects of CS and TBCC on growth performance of pigs are summarized in Table 2. There were no significant differences between the CS and TBCC diets for growth performance, but there was a tendency for decreased ADG during d 1 to 81 in pigs fed the TBCC diet compared with pigs fed the CS diet (p<0.10).
On d 30, serum total protein, albumin, urea, and ALT were not affected by Cu sources, but activities of serum AST in pigs fed the TBCC diet were higher than that in pigs fed the CS diet (p<0.05; Table 3). On d 81, serum total protein, albumin, urea, and AST were not different between pigs fed the CS diet and TBCC diet, but the activities of serum ALT in pigs fed the TBCC diet trended to higher than that in pigs fed the CS diet (p<0.1). Data on the concentrations of Cu in serum are shown in Table 3. There were no differences in the levels of Cu in serum between the CS and TBCC treatments.
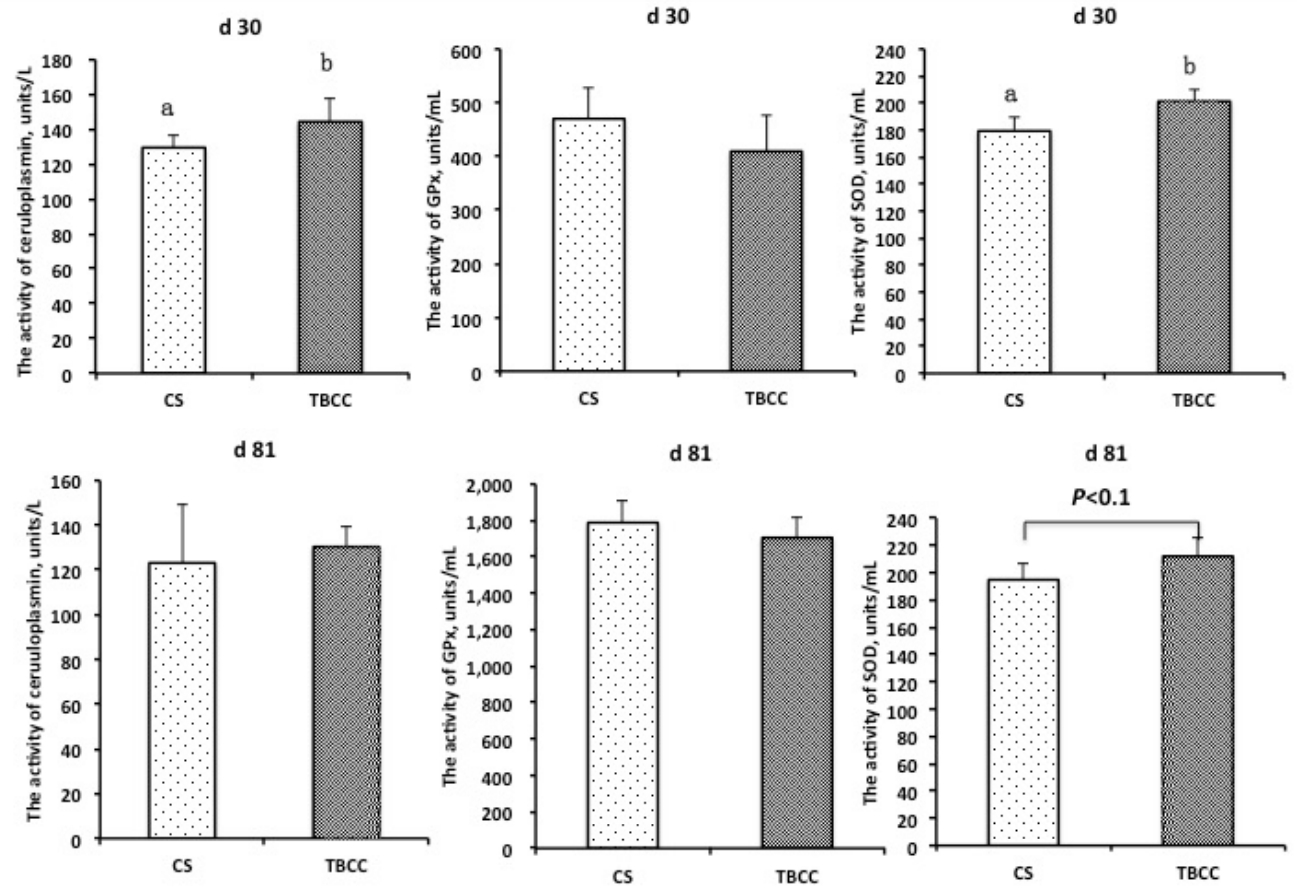
As shown in Figure 1, TBCC significantly increased the activities of ceruloplasmin and SOD in serum compared with CS on d 30 (p<0.05). There was a tendency for increased activity of SOD in pigs fed the TBCC diet compared with pigs fed the CS diet on d 81 (p<0.1). However, there was no difference between the TBCC diet and the CS diet for the activity of GPx.
Data for Cu, Fe, Zn, and Mn concentrations in tissues when supplementing the CS or TBCC diet with 130 mg/kg Cu for 81 d are presented in Table 4. Copper level were 20 to 40 mg/kg in liver and kidney and less than 10 mg/kg in muscle in both CS and TBCC treatments on d 81. The TBCC produced a significantly lower Cu level in the liver on d 81 compared with CS (p<0.05). No significant differences in Cu deposition in the kidney and muscle were observed between CS and TBCC. The Cu sources had no effects on the concentrations of Fe, Mn, and Zn in the liver, kidney, and muscle on d 81.
On d 102, when the supplementation level of Cu was decreased to 10 mg/kg for 21 d, TBCC increased Mn level in the liver (p<0.05; Table 5). No significant differences in Cu deposition in the liver, kidney, and muscle were observed between CS and TBCC. The Cu sources had no significant effect on Fe and Zn deposition in the liver, kidney, and muscle on d 102.
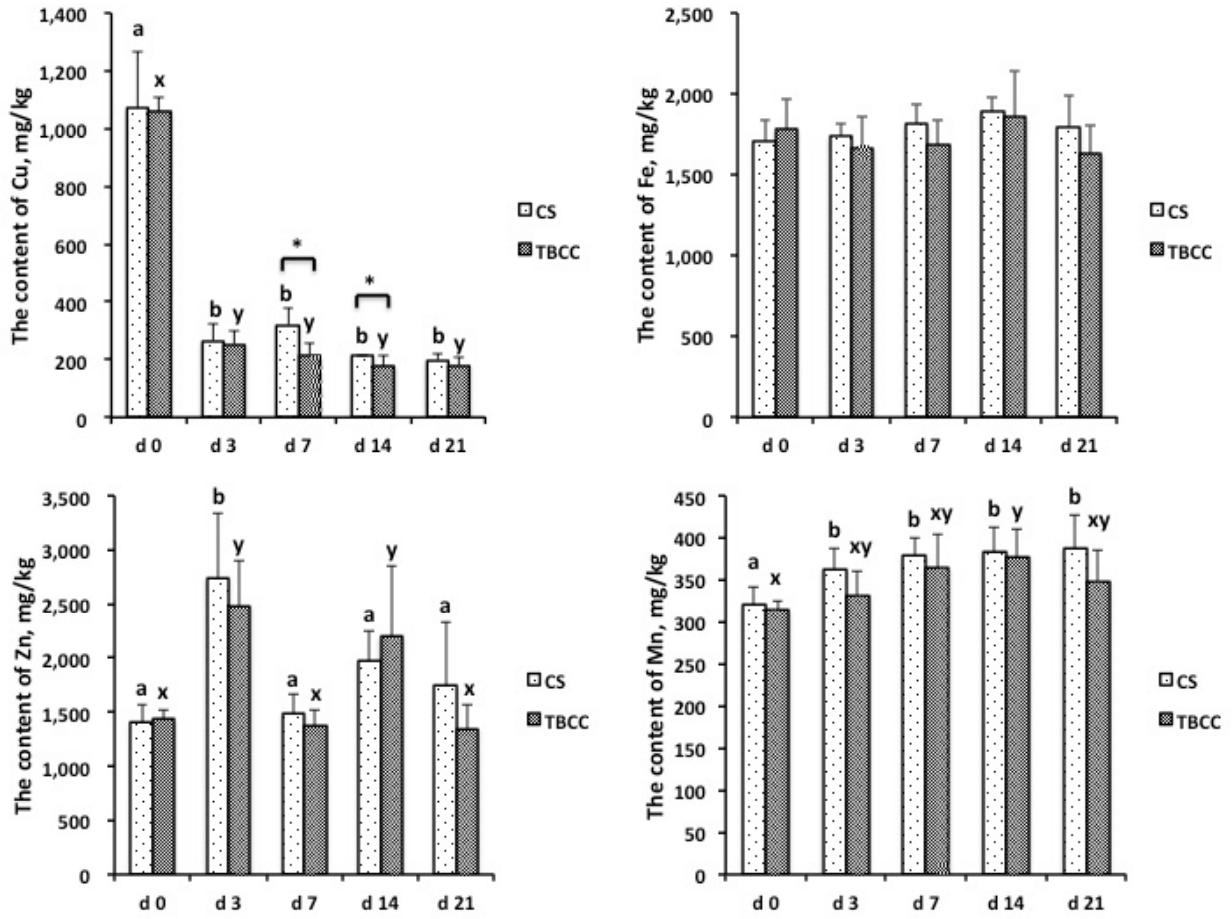
The concentrations of Cu in diets significantly influenced the level of Cu in feces (Figure 2). The concentrations of Cu in feces sharply decreased when the supplemental Cu level in diet changed from 130 mg/kg to 10 mg/kg (p<0.05). The sources of Cu in the diet also influenced the level of Cu in feces on d 7 and 14 after the supplemental Cu level was changed to 10 mg/kg with pigs fed TBCC having decreased concentrations of Cu in feces compared to those fed CS (p<0.05).
From Figure 2, we also found that decreasing the level of Cu in the diet increased the concentration of Zn and Mn in feces. Compared with d 0, the level of Zn in feces increased in the CS diet at d 3 as well as the TBCC diet at d 3 and 14 (p<0.05). The concentrations of Mn in feces in the CS treatment were increased at d 3, 7, 14, and 21 compared with d 0 (p<0.05). The concentration of Mn in feces in the TBCC treatment was increased at d 14 compared with d 0 (p<0.05). There was no difference in the concentrations of Fe, Zn, and Mn in feces between the CS diet and the TBCC diet during d 0 to 21 after the supplemental Cu level was changed from 130 mg/kg to 10 mg/kg.
High levels of Cu are commonly used as growth promoters in the swine industry and its benefits on weight gain and efficiency of feed utilization in both weaned pigs and finishing pigs have been well documented [3,9]. The TBCC chloride has been studied as a Cu source for animal production in pigs. Previous studies reported that TBCC was as effective as CS at improving growth in weanling and finishing pigs [10,11]. In the present study, there was no significant difference on growth performance between pigs fed diets with 130 mg/kg Cu from TBCC or CS, but TBCC had a tendency to decrease ADG of pigs compared with CS during the first 81 d. However, although the purpose of this study was to investigate the difference of 2 main inorganic copper sources, CS and TBCC, on growth performance, physiochemical parameters, deposition and excretion, it would have been better include a low Cu diet group in this experiment. In that case, we could have determined the effects of 130 mg/kg Cu on growth performance of growing-finishing pigs.
The AST and ALT are 2 important transaminase enzymes, and their concentrations in serum are commonly measured as a part of a diagnostic evaluation of liver function [12]. When hepatic cells are damaged or cell membrane permeability increased, ALT and AST in the liver permeates into the blood, which results in an increase of these enzymesŌĆÖ activity in serum [13]. Previous research reported that the concentrations of AST and ALT in the plasma of rats were significantly elevated by dietary Cu overload [14]. Pu et al [15] showed that the concentrations of AST and ALT in serum of finishing pigs, when fed a diet with NRC level of CS, were 5.87 and 10.20 U/L, respectively. Compared with PuŌĆÖs results, our study showed that 130 mg/kg Cu, whether sourced from CS or TBCC, significantly increased serum concentrations of AST and ALT. At the same time, we measured the main antioxidant enzymes GPx, SOD, and ceruloplasmin in serum and found that TBCC significantly increased the activity of SOD and ceruloplasmin in serum on d 30 compared with CS. Ceruloplasmin is not only the transport protein, which is responsible for carrying Cu to tissues that need the mineral, and an enzyme catalyzing the oxidation of minerals but also an important extracellular antioxidant and free radical scavenger [16]. Chronic Cu overload could initiate oxidative stress [17]. The enhancement of the activities of antioxidant enzymes could be the results of mitigating oxidative tissue damage [18]. Another study showed that oxidative stress was always coupled with higher serum AST and ALT levels [19]. In this study, pigs fed the TBCC diet with 130 mg/kg Cu for 30 d had higher levels of AST as well as SOD and ceruloplasmin than CS diet, which might indicate that TBCC diet caused more oxidative stress than CS diet. However, the result was inconsistent with previous result that TBCC might cause less oxidative stress in duodenum of weanling pigs than CS when fed at 225 mg Cu/kg diet for 11/12 d [20]. The possible reasons could be the difference of pigsŌĆÖ age, the length of feeding time and the level of dietary Cu.
Previous studies reported that liver Cu concentrations linearly increased with the increase of Cu concentrations in both TBCC and CS diets [21]. From the results of this experiment, we observed that the concentration of Cu in the liver and kidney were 2 to 3 times higher than 10 mg/kg, which is the maximum permitted level of Cu in food according to the hygienic standard for meat products of Chinese Regulation [4], after long-term supplementation with 130 mg/kg dietary Cu from both TBCC and CS treatments. Furthermore, decreasing the supplementation level of Cu in both TBCC and CS diets to 10 mg/kg for 21 d still did not decrease the content of Cu in organs to less than 10 mg/kg. In this study, we also found that liver Cu was significantly higher in CS pigs than TBCC pigs when fed at 130 mg Cu/kg diet. This result agreed with previous result that TBCC diet significantly decreased the concentration of Cu in the liver of weanling pigs when fed at 225 mg Cu/kg diet [22].
Copper is known to react with a variety of nutrients, and the interactions of microelements are very complicated and can affect the absorption and bioavailability of other nutrients. Previous research indicated that Fe, Cu, and Zn affected the uptake of one another by possible competitive inhibition of transport and bioavailability [23]. Copper and Mn are the important components of SOD [24]. In this study, we also found that TBCC significantly increased Mn concentration in the liver compared with CS. The possible mechanism may be competitive binding to the divalent metal transporter 1 protein, which participates in divalent metal transport (Fe, Cu, Zn, Mn, and Pb) [25]. Divalent metal transporter 1 represents a large family of metal ion transporters that are highly conserved from bacteria to humans [8]. The results of this study indicated that Cu from TBCC or CS has different effects on Mn metabolism. However, the mechanisms of the influence on metabolism of Zn and Mn by these 2 Cu sources need further research.
The Cu contents of pigsŌĆÖ feces are largely a reflection of the concentration in the feeds consumed by the pigs. Studies showed that pigs absorbed only about 5% to 10% dietary Cu or even less [26]. Therefore, a high level of Cu will be excreted through the feces, which would result in excessive accumulation of Cu in the soils where the manure is applied and enrichment of Cu in plants that might induce a potential health risk to livestock or humans [27,28]. From the results of this study, we found that the concentrations of Cu in feces sharply decreased from 1,064 mg/kg DM to 259 mg/kg DM from d 0 to 3 when the supplemental levels of Cu in the diets changed from 130 mg/kg to 10 mg/kg. Copper contents in feces had already exceeded the maximum permitted limit of Cu (250 mg/kg) in China [29]. Residues of Cu in manures can accumulate in surface soils, which could increase the risk of Cu contamination in the environment [30]. Recent research found that applications of pig manure containing high doses of Cu may lead to enrichment of Cu in brown rice and have potential long-term health risks through the food chain [31]. Excessive exposure to Cu has been linked to cellular damage in humans [32]. From the aspects of environmental protection and the safety of livestock and humans, Cu levels in animal feed should be controlled based on the relevant legal limits in China. According to Chinese regulation, Cu level in piglets, growing and finishing pigs which BWs were less than 30, 30 to 60 and greater than 60 kg, are not allowed to exceed the maximum permitted limit level 200, 150, 35 mg/kg, respectively. Therefore, the results of this experiment reminded us that as more Cu was added to the diet, the amount accumulated in organs and the amount excreted by the animal also increased with both copper sources, SC and TBCC. So, to avoid the potential adverse effects of a high level of Cu on the characteristics of animal products and ecological environment, it is important to use only the recommended dietary levels of Cu in swine production.
In conclusion, the present experiment suggested that TBCC had no significant difference on growth performance but had higher activities of AST, ceruloplasmin and SOD than CS in growing pigs when pigs were fed diets with 130 mg Cu/kg diet for 30 d. At the same time, TBCC led to a lower accumulation of Cu in the liver of growing pigs than CS when diet was supplemented with 130 mg/kg Cu for 81 d. Cu concentrations in feces were significantly influenced by the concentration of Cu in both TBCC and CS diets.
ACKNOWLEDGMENTS
This study was founded by the earmarked fund from the China Pig Modern Industrial Technology System Grant (CARS-35) and the Science and Technology Support Program of Sichuan Province (2016NZ0006).
Figure┬Ā1
Effect of copper sulfate (CS) and tribasic copper chloride (TBCC) on serum activities of ceruloplasmin, glutathione peroxidase (GPx), and superoxide dismutase (SOD) in pigs. a,b Means with different superscript letters represent differences between the CS diet and the TBCC diet (p<0.05).
Figure┬Ā2
The concentrations of Cu, Fe, Zn, and Mn in feces of pigs when the supplementation Cu level changed from 130 mg/kg to 10 mg/kg with the copper sulfate (CS) or tribasic copper chloride (TBCC) diet at d 0, 3, 7, 14, and 21 (mg/kg dry matter). Means with different superscript letters represent differences between different time points within the CS diet (aŌĆōc) or the TBCC diet (xŌĆōz; p<0.05). * p<0.05, CS vs TBCC.
Table┬Ā1
Ingredients and nutrient compositions of basal diets (DM basis)
| Item | Phase 11) | Phases 2 and 31) |
|---|---|---|
| Ingredients (%) | ||
| ŌĆāCorn | 69.71 | 73.62 |
| ŌĆāWheat bran | 5.00 | 6.30 |
| ŌĆāSoybean meal | 19.00 | 16.00 |
| ŌĆāFish meal | 2.00 | 0.00 |
| ŌĆāMonocalcium phosphate | 0.80 | 0.70 |
| ŌĆāLimestone | 0.90 | 0.90 |
| ŌĆāSoybean oil | 1.80 | 1.80 |
| ŌĆāL-lysine HCl | 0.15 | 0.04 |
| ŌĆāSalt | 0.30 | 0.30 |
| ŌĆāTrace mineral premix2) | 0.20 | 0.20 |
| ŌĆāVitamin premix3) | 0.04 | 0.04 |
| ŌĆāCholine chloride | 0.10 | 0.10 |
| Nutrients composition | ||
| ŌĆāDE4) (Mcal/kg) | 13.97 | 13.97 |
| ŌĆāCP5) (%) | 15.56 | 13.30 |
| ŌĆāCa5) (%) | 0.67 | 0.55 |
| ŌĆāTotal P5) (%) | 0.54 | 0.42 |
| ŌĆāAvailable P4) (%) | 0.33 | 0.26 |
| ŌĆāDigestible Lys4) (%) | 0.92 | 0.66 |
| ŌĆāDigestible Met+Cys4) (%) | 0.58 | 0.44 |
| ŌĆāDigestible Thr4) (%) | 0.60 | 0.51 |
| ŌĆāDigestible Trp4) (%) | 0.17 | 0.15 |
DM, dry matter; DE, digestible energy; CP, crude protein; Ca, calcium; P, phosphorus; Lys, lysine; Met, methionine; Cys, cystine; Thr, threonine; Trp, tryptophan.
1) Body weight range and the duration of phase 1, phase 2, and phase 3 were 30 to 50 kg (d1 to 30), 50 to 100 kg (d 31to 81), and 100 to 115 kg (d 82 to 102), respectively.
2) Mineral premix provided the following amounts per kg complete diet: 120 mg Fe (FeSO4┬Ę7H2O), 120 mg Zn (ZnSO4┬Ę7H2O), 20 mg Mn (MnSO4┬ĘH2O), 0.3 mg Se (Na2SeO3┬Ę5H2O), and 0.3 mg I (KI).
Table┬Ā2
Effects of copper sulfate (CS) and tribasic copper chloride (TBCC) on the growth performance in pigs
Table┬Ā3
Effect of copper sulfate (CS) and tribasic copper chloride (TBCC) on the physiochemical parameters and Cu concentration in serum of pigs
Table┬Ā4
Effects of copper sulfate (CS) and tribasic copper chloride (TBCC) on the concentration of Cu, Fe, Zn, and Mn in tissues on d 81 (mg/kg dry matter)
Table┬Ā5
Effect of copper sulfate (CS) and tribasic copper chloride (TBCC) on the concentrations of Cu, Fe, Zn, and Mn in tissues on d 102 (mg/kg dry matter)
REFERENCES
1. NRC. Nutrient requirements of swine, eleventh reved. Washington, DC, USA: National Academy Press; 2012.
2. Coble KF, DeRouchey JM, Tokach MD, et al. The effects of copper source and concentration on growth performance, carcass characteristics, and pen cleanliness in finishing pigs. J Anim Sci 2017; 95:4052ŌĆō9.


3. Braude R. Copper as a stimulant in pig feeding. World Rev Anim Prod 1967; 3:69ŌĆō82.
4. China GB. Tolerance limit of copper in foods. Beijing, China: China Standards Press; 1994.
5. Park CS, Kim BG.
In vitro solubility of copper (II) sulfate and dicopper chloride trihydroxide for pigs. Asian-Australas J Anim Sci 2016; 29:1608ŌĆō15.




6. Feldpausch JA, Amachawadi RG, Scott HM, et al. Effects of added copper and zinc on growth performance and carcass characteristics of finishing pigs fed diets with or without ractopamine HCl. Kansas, USA: Kansas Agricultural Experiment Station Research Reports; 2015. 1:p. 710.4148/2378-5977.1134
7. AOAC. Official methods of analysis. 16th edition. Association of Official Analytical Chemists. Washington, DC, USA: AOAC International; 1995.
8. Cellier M, Priv├® G, Belouchi A, et al. Nramp defines a family of membrane proteins. Proc Natl Acad Sci USA 1995; 92:10089ŌĆō93.



9. Hill GM, Cromwell GL, Crenshaw TD, et al. Growth promotion effects and plasma changes from feeding high dietary concentrations of zinc and copper to weanling pigs (regional study). J Anim Sci 2000; 78:1010ŌĆō6.


10. Clegg MS, Keen CL, L├Čnnerdal B, Hurley LS. Influence of ashing techniques on the analysis of trace elements in animal tissue. Biol Trace Elem Res 1981; 3:107ŌĆō15.


11. Cromwell GL, Lindemann MD, Monegue HJ, Hall DD, Orr DE. Tribasic copper chloride and copper sulfate as copper sources for weanling pigs. J Anim Sci 1998; 76:118ŌĆō23.


12. Ronald L, Koretz MD. Chronic hepatitis: science and superstition. Cur Hepatol 1992; 12:53ŌĆō74.
13. He J, Zhang KY, Chen DW, et al. Effects of maize naturally contaminated with aflatoxin B1 on growth performance, blood profiles and hepatic histopathology in ducks. Livest Sci 2013; 152:192ŌĆō9.

14. Sokol RJ, Devereaux M, Mierau GW, Hambidge KM, Shikes RH. Oxidant injury to hepatic mitochondrial lipids in rats with dietary copper overload modification by vitamin E deficiency. Gastroenterology 1990; 99:1061ŌĆō71.


15. Pu J, Tian G, Li B, et al. Trace mineral overload induced hepatic oxidative damage and apoptosis in pigs with long-term high-level dietary mineral exposure. J Agric Food Chem 2016; 64:1841ŌĆō9.


16. Yang F, Friedrichs W, deGraffenried L, et al. Cellular expression of ceruloplasmin in lung: Antioxidant role. Am J Res Cell Mol 1996; 14:161ŌĆō9.

17. Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003; 189:147ŌĆō63.


18. Obrosova IG, Stevens MJ. Effect of dietary taurine supplementation on GSH and NAD (P)-redox status, lipid peroxidation, and energy metabolism in diabetic precataractous lens. Invest Ophthalmol Vis Sci 1999; 40:680ŌĆō8.

19. Huang GJ, Deng JS, Huang SS, et al. Protective effect of antrosterol from antrodia cam phorata submerged whole broth against carbon tetrachloride-induced acute liver injury in mice. Food Chem 2012; 132:709ŌĆō16.

20. Huang YL, Ashwell MS, Fry RS, et al. Effect of dietary copper amount and source on copper metabolism and oxidative stress of weanling pigs in short-term feeding. J Anim Sci 2015; 93:2948ŌĆō55.



21. Miles RD, OŌĆÖKeefe SF, Henry PR, Ammerman CB, Luo XG. The effect of dietary supplementation with copper sulfate or tribasic copper chloride on broiler performance, relative copper bioavailability, and dietary prooxidant activity. Poult Sci 1998; 77:416ŌĆō25.



22. Fry RS1, Ashwell MS, Lloyd KE, et al. Amount and source of dietary copper affects small intestine morphology, duodenal lipid peroxidation, hepatic oxidative stress, and mRNA expression of hepatic copper regulatory proteins in weanling pigs. J Anim Sci 2012; 90:3112ŌĆō9.



23. Araya M, Pizarro F, Olivares M, Arredondo M, Gonzalez M. Understanding copper homeostasis in humans and copper effects on health. Biol Res 2006; 39:183ŌĆō7.


24. Antonyuk SV, Strange RW, Marklund SL, Hasnain SS. The structure of human extracellular copper-zinc superoxide dismutase at 1.7 A resolution: Insights into heparin and collagen binding. J Mol Biol 2009; 388:310ŌĆō26.


25. Garrick MD, Dolan KG, Horbinski C, et al. DMT1: A mammalian transporter for multiple metals. Biometals 2003; 16:41ŌĆō54.


26. Jondreville C, Revy PS, Dourmad JY. Dietary means to better control the environmental impact of copper and zinc by pigs from weaning to slaughter. Livest Prod Sci 2003; 84:147ŌĆō56.

27. Li L, Xu Z, Wu J, Tian G. Bioaccumulation of heavy metals in the earthworm Eisenia foetida in relation to bioavailable metal concentrations in pig manure. Bioresour Technol 2010; 101:3430ŌĆō6.


28. Qureshi A, Lo KV, Liao PH, Mavinic DS. Real-time treatment of dairy manure: implications of oxidation reduction potential regimes to nutrient management strategies. Bioresour Technol 2008; 99:1169ŌĆō76.


29. China GB 4284-84. Control standards for pollutants in sludges fromagricultural use. Beijing, China: China Standards Press; 1984.
30. Guan TX, He HB, Zhang XD, Bai Z. Cu fractions, mobility and bioavailability in soil-wheat system after Cu-enriched livestock manure applications. Chemosphere 2011; 82:215ŌĆō22.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





