 |
 |
| Anim Biosci > Volume 30(12); 2017 > Article |
|
Abstract
Objective
In the present study, we examined whether the post-prandial reduction in plasma growth hormone (GH) levels is related to the increase in plasma insulin levels in ruminants.
Methods
We performed two experiments: intravenous bolus injection of insulin (0.2 IU/kg body weight) or glucose (1.0 mmol/kg body weight) was administered to increase the plasma insulin levels in male Shiba goats.
Results
In the insulin injection experiment, significant (p<0.05) increase in GH concentrations was observed, 15 to 20 min after the injection; it was accompanied with a significant (p<0.01) increase in cortisol concentrations at 45 to 90 min, when compared to the concentrations in the saline-injected controls. The glucose injection significantly (p<0.05) increased the plasma GH concentration at 20 to 45 min; this was not accompanied by significantly higher cortisol concentrations than were observed for the saline-injected control. Hypoglycemia induced by the insulin injection, which causes the excitation of the adrenal cortex, might be involved in the increase in insulin levels.
Feeding has been reported to induce reduction in the concentration of growth hormone (GH) in plasma [1,2], and cause growth hormone-releasing hormone (GHRH)-induced increase in GH in ruminant animals [1,3]. We hypothesized that post-prandial decrease in GH levels may be caused by increase in the levels of plasma short-chain fatty acids (SCFA), resulting from increased rumen fermentation and subsequent absorption of SCFA in the blood. This hypothesis was partly supported by reports showing that infusion of SCFA into blood, and/or an increase of SCFA in the medium bathing the anterior pituitary gland cells, suppressed GHRH-induced GH release [4–6]. However, it was also reported that an increase in mesenteric SCFA concentrations, which was mimicked by the infusion of mixed SCFA up to the post-prandial level, was not enough to suppress GHRH-induced GH increase [7]. From these results, it appears that physiological increase in plasma SCFA levels after feeding might not be enough to suppress the GH levels, highlighting the need for another explanation for these phenomena.
It is known that feeding decreases plasma GH, but increases plasma insulin levels, which indicates a reciprocal relationship between these two major metabolic hormones in adult ruminant animals [2]. In the adults of some animal species, a relationship between plasma GH and insulin concentrations has been reported [8–13]. Therefore, the post-prandial increase in plasma insulin concentration, which is usually accompanied by an increase in glucose concentration, might inhibit GH release from the anterior pituitary gland of the ruminant.
In the present study, we examined an alternative hypothesis for the post-prandial reduction in plasma GH levels: whether increase in plasma insulin levels is involved in GH reduction mechanisms. To accomplish this objective, we performed two experiments: intravenous infusion of insulin or glucose to increase the plasma insulin levels in goats.
Male Shiba goats were treated in accordance with the “Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences”, as recommended by The Physiological Society of Japan. The experimental procedures were approved by the Animal Care Committee of Tohoku University (Approval number: 2010AgA-24).
The animals were fed alfalfa hay cubes (1.3%/total body weight [BW]) at 1000 h in the morning and were offered water and mineral salts ad libitum for a week before the blood sampling day. The nutrient composition of Alfalfa hay cubes (dry matter 87.5%) was crude protein 16.9%, ether extract 1.5%, acid detergent fiber 30.5%, neutral detergent fiber 39.1%. Alfalfa hay cubes were removed 16 hours before the blood sampling.
Five male goats (23.28±3.52 kg BW) were used in this experiment. A polyethylene catheter was inserted into the left jugular vein of the animals 2 h prior to blood sampling for stabilization and reducing stress as our previous reports [14,15]. No acute increment of non-esterified fatty acid (NEFA) and cortisol before blood sampling indicated that 2 h is enough for stabilization in goats (Figures 1, 2). The catheter was kept in sterile 3.5% tri-sodium citrate. The blood was sampled from 0900 to 1300 h. Approximately 5 mL of blood was collected at each sampling time, 30 or 60 min before and 120 min after bolus injection of insulin (0.2 IU/kg BW), glucose (1.0 mmol/kg BW), or saline into venous blood through the catheter. All blood samples were kept in an ice bath until centrifugation at 3,000×g for 10 min at 4°C. The separated plasma samples were stored at −20°C until the analysis.
The concentrations of GH and insulin in the plasma were determined by radioimmunoassay, as described previously [16–18]. Cortisol concentration in plasma was analyzed by Cortisol ELISA kit (Enzo Life Sciences, Inc., Farmingdale, NY, USA). The plasma glucose and NEFA concentrations were measured using a commercial kit (Glucose C-II Test-Wako, NEFA C-Test-Wako; Wako Pure Chemical Co., Tokyo, Japan).
Compared to the control injection, the insulin injection, which significantly increased the plasma GH concentrations after 15 and 20 min (Figure 1A), significantly increased the plasma insulin concentrations 5 min and 30 to 60 min after the injection (Figure 1B). The blood glucose concentrations were significantly lower after 20 to 120 min (Figure 1C), whereas the concentrations of NEFA, 45 to 90 min after the injection were significantly higher, than the concentrations observed in the control (Figure 1D).
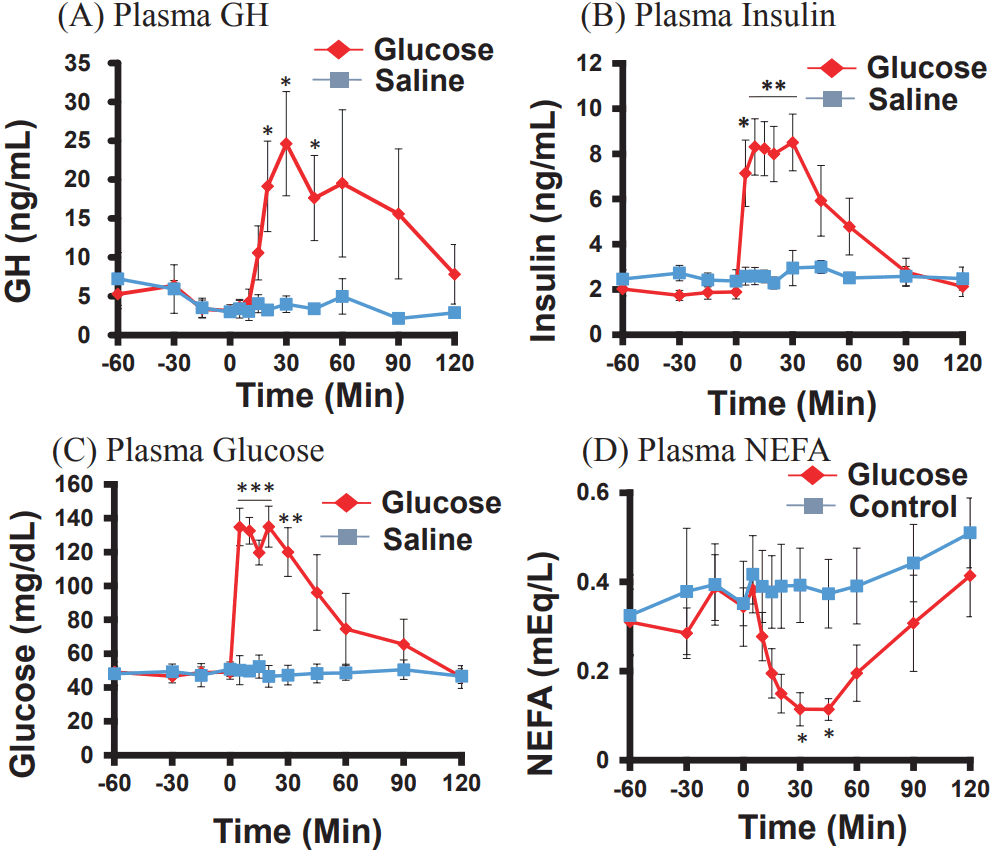
The concentrations of plasma GH were significantly higher than those in the control, 20 to 45 min after the glucose injection (Figure 2A). The plasma insulin concentrations were also significantly higher, 5 to 30 min after the injection, than in the control (Figure 2B). The blood glucose concentrations were immediately increased at 5 to 30 min (Figure 2C), whereas the plasma NEFA concentrations decreased, gradually but significantly, 30 and 45 min after the injection, as compared to the decrease in the control (Figure 2D).
The present study demonstrated that venous injection of insulin or glucose immediately and significantly increased the plasma concentrations of GH. This fact indicates that increase in plasma insulin concentrations always causes a rise in plasma GH concentrations, and that plasma insulin or glucose levels are not related to the post-prandial reduction in GH levels. The doses of insulin and glucose injection used in the present study have been employed in previous studies [19,20].
Volatile fatty acids increased after feeding have the inhibitory effects on GH secretion, and stimulatory effects on insulin secretion in sheep [2,6,7]. However, the significant increase in GH concentrations after the insulin infusion might have been caused by the stress from hypoglycemia and consequent stimulation of the hypothalamus-pituitary-adrenal (HPA) axis, because plasma cortisol concentrations were significantly increased by the insulin injection (Figure 3A). We previously reported that excitation of the HPA axis caused an increase in plasma GH concentrations [15,17]. Apelin and arginine vasopressin (AVP), the key stress hormones in ruminants, were reported to stimulate the secretion of adrenocorticotropic hormone [21–23], and subsequently cortisol was also demonstrated to stimulate GH secretion [14,17]. Lipopolysaccharide injection stimulated GH secretion in rats [24] and corticotropin-releasing hormone injection had the same effect in humans [25]. There are the possibilities on apelin and AVP secretions by insulin injection in the present experiment. All these findings suggest that stimulation of the HPA axis induced by insulin injection might be involved in the increased GH secretion. Further studies are needed to clearly demonstrate the action of insulin on GH secretion in hypothalamus and pituitary gland.
It also appears that insulin-induced hypoglycemia was involved in the elevation of GH concentrations. Insulin-induced hypoglycemia causes an increase in GH concentrations in humans [26]. It was also reported that restricted-feeding increased GH levels in ewes [27], suggesting that low-glucose conditions might have raised the plasma GH concentrations in the present study, although such a physiological phenomenon was not observed in sheep [28]. As GH acts to promote the release of glucose from liver through activation of the Janus kinase 2/Signal transducer and activator of transcription 5 (JAK2/STAT5) pathway in mice [29], it is possible that GH release was stimulated by insulin-induced hypoglycemia to aid the recovery of the lowered blood glucose levels.
The mechanism for increased GH concentrations induced by the glucose injection might be different from the mechanism of increase induced by the insulin injection, because plasma cortisol concentrations were not changed by the glucose injection. It was reported that glucose infusion induced an increase in plasma GH concentrations in dairy cows [30,31] and glucose stimulated the release of GH in goat anterior pituitary cells in vitro [32,33].
The NEFA was decreased immediately after the insulin injection, but it rapidly increased thereafter. This change was reciprocally accompanied by that of glucose concentrations (Figures 1, 2). Insulin promotes lipogenesis by increasing NEFA uptake by the adipose tissues [34]; however, GH has the opposite action [35]. It also appears that changes in plasma NEFA, as well as in glucose, might be involved in changing GH concentrations, because it has been reported that fatty acids directly inhibit GH secretion from cultured anterior pituitary cells [4].
In conclusion, GH secretion was significantly elevated, and not reduced, by the bolus venous injection of insulin as well as of glucose. Insulin, not glucose, also stimulated an increase in cortisol concentrations, indicating that increased GH secretion might be caused by stimulation of the HPA axis. Factors other than insulin might be involved in the post-prandial GH reduction in goats.
Figure 1
Effects of intravenous injection of insulin (0.2 IU/kg body weight) or saline on plasma concentrations of growth hormone (GH) (A), insulin (B), glucose (C), and non-esterified fatty acid (NEFA) (D) in goats (n = 5). The values are represented as mean±standard error of the mean. * p<0.05; ** p<0.01; *** p<0.001 (control vs insulin, unpaired Student’s t-test).
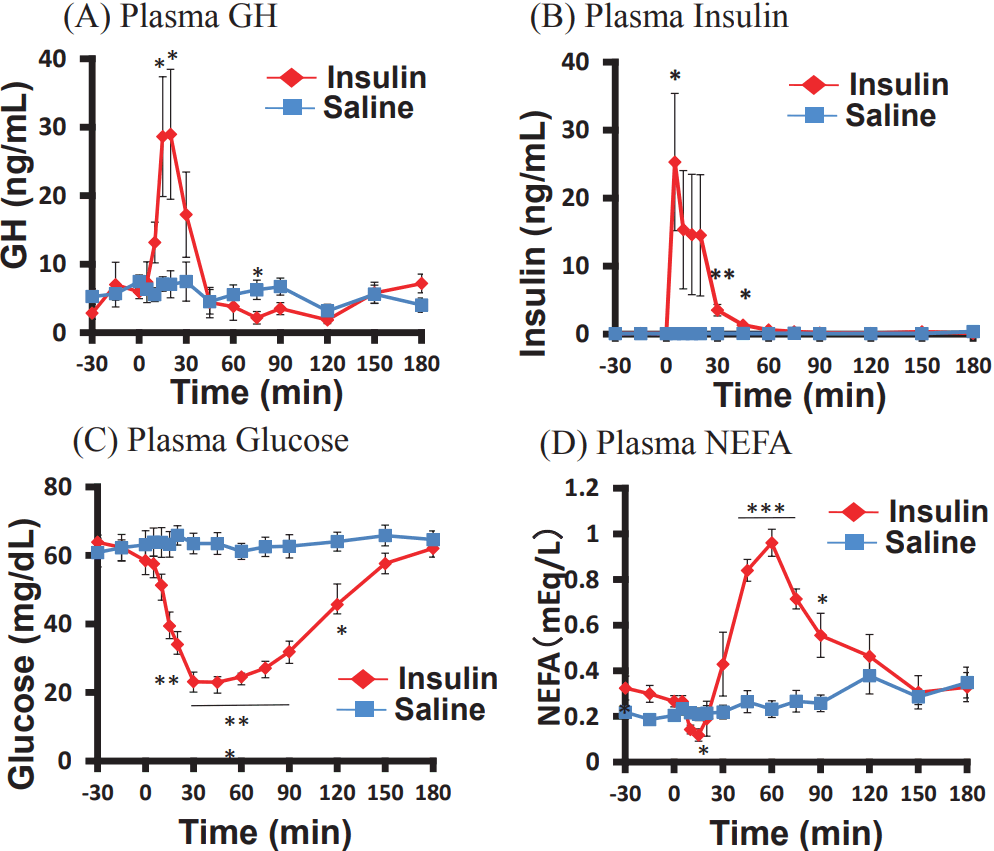
Figure 2
Effects of intravenous injection of glucose (1.0 mmol/kg body weight) or saline on plasma concentrations of growth hormone (GH) (A), insulin (B), glucose (C), and non-esterified fatty acid (NEFA) (D) in goats (n = 4). The values are represented as mean±standard error of the mean. * p<0.05; ** p<0.01; *** p<0.001 (control vs insulin, unpaired Student’s t-test).
REFERENCES
1. Moseley WM, Alaniz GR, Claflin WH, Krabill LF. Food intake alters the serum growth hormone response to bovine growth hormone-releasing factor in meal-fed Holstein steers. J Endocrinol 1988; 117:253–9.


2. Matsunaga N, Arakawa NT, Goka T, et al. Effects of ruminal infusion of volatile fatty acids on plasma concentration of growth hormone and insulin in sheep. Domest Anim Endocrinol 1999; 17:17–27.


3. McMahon CD, Chapin LT, Lookingland KJ, Radcliff RP, Tucker HA. Feeding reduces activity of growth hormone-releasing hormone and somatostatin neurons. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine 2000; 223:210–7.

4. Ishiwata H, Nagano M, Sasaki Y, Chen C, Katoh K. Short-chain fatty acids inhibit the release and content of growth hormone in anterior pituitary cells of the goat. Gen Comp Endocrinol 2000; 118:400–6.


5. Katoh K, Ohata Y, Ishiwata H. Suppressing effects of short-chain fatty acids on growth hormone (GH)-releasing hormone-induced GH release in isolated anterior pituitary cells of goats. Domest Anim Endocrinol 1999; 17:85–93.


6. Matsunaga N, Nam KT, Kuhara T, et al. Inhibitory effect of volatile fatty acids on GRF-induced GH secretion in sheep. Endocr J 1993; 40:529–37.


7. Matsunaga N, Kubota I, Roh SG, et al. Effect of mesenteric venous volatile fatty acids (VFA) infusion on GH secretion in sheep. Endocr J 1997; 44:707–14.


8. Gahete MD, Cordoba-Chacon J, Lin Q, et al. Insulin and IGF-I inhibit GH synthesis and release in vitro and in vivo by separate mechanisms. Endocrinology 2013; 154:2410–20.




9. Luque RM, Kineman RD. Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology 2006; 147:2754–63.


10. De Marinis L, Bianchi A, Mancini A, et al. Growth hormone secretion and leptin in morbid obesity before and after biliopancreatic diversion: relationships with insulin and body composition. J Clin Endocrinol Metab 2004; 89:174–80.


11. Lanzi R, Luzi L, Caumo A, et al. Elevated insulin levels contribute to the reduced growth hormone (GH) response to GH-releasing hormone in obese subjects. Metab: Clin Exp 1999; 48:1152–6.

12. Melmed S. Insulin suppresses growth hormone secretion by rat pituitary cells. J Clin Invest 1984; 73:1425–33.



13. Yamashita S, Melmed S. Effects of insulin on rat anterior pituitary cells. Inhibition of growth hormone secretion and mRNA levels. Diabetes 1986; 35:440–7.


14. Roh SG, Koiwa K, Sato K, et al. Actions of intravenous injections of AVP and oxytocin on plasma ACTH, GH, insulin and glucagon concentrations in goats. Anim Sci J 2014; 85:286–92.


15. Sato K, Takahashi T, Kobayashi Y, et al. Apelin is involved in postprandial responses and stimulates secretion of arginine-vasopressin, adrenocorticotropic hormone, and growth hormone in the ruminant. Domest Anim Endocrinol 2012; 42:165–72.


16. Suzuki Y, Song SH, Sato K, et al. Chemerin analog regulates energy metabolism in sheep. Anim Sci J 2012; 83:263–7.


17. Katoh K, Yoshida M, Kobayashi Y, et al. Responses induced by arginine-vasopressin injection in the plasma concentrations of adrenocorticotropic hormone, cortisol, growth hormone and metabolites around weaning time in goats. J Endocrinol 2005; 187:249–56.


18. Katoh K, Asari M, Ishiwata H, Sasaki Y, Obara Y. Saturated fatty acids suppress adrenocorticotropic hormone (ACTH) release from rat anterior pituitary cells in vitro
. Comp Biochem Physiol A Mol Integr Physiol 2004; 137:357–64.


19. Caraty A, Grino M, Locatelli A, et al. Insulin-induced hypoglycemia stimulates corticotropin-releasing factor and arginine vasopressin secretion into hypophysial portal blood of conscious, unrestrained rams. J Clin Invest 1990; 85:1716–21.



20. Fukumori R, Mita T, Sugino T, et al. Effects of glucose and volatile fatty acids on blood ghrelin concentrations in calves before and after weaning. J Anim Sci 2012; 90:4839–45.


21. Familari M, Smith AI, Smith R, Funder JW. Arginine vasopressin is a much more potent stimulus to ACTH release from ovine anterior pituitary cells than ovine corticotropin-releasing factor. 1. In vitro studies. Neuroendocrinology 1989; 50:152–7.


22. Fora MA, Butler TG, Rose JC, Schwartz J. Adrenocorticotropin secretion by fetal sheep anterior and intermediate lobe pituitary cells in vitro: effects of gestation and adrenalectomy. Endocrinology 1996; 137:3394–400.



23. Hasegawa N, Sugiwaka T, Kawamura T, Katoh K. Changes in serum ACTH and cortisol concentrations after administration of arginine-vasopressin (AVP) in dairy cattle. Anim Sci Technol 1996; 67:591–2.

24. Priego T, Granado M, Ibanez de Caceres I, et al. Endotoxin at low doses stimulates pituitary GH whereas it decreases IGF-I and IGF-binding protein-3 in rats. J Endocrinol 2003; 179:107–17.


25. Raza J, Massoud AF, Hindmarsh PC, Robinson IC, Brook CG. Direct effects of corticotrophin-releasing hormone on stimulated growth hormone secretion. Clin Endocrinol 1998; 48:217–22.

26. Hanew K. The mechanism of arginine- and insulin-induced GH release in humans. Endocr J 2000; 47:SupplS23–7.


27. Thomas GB, Mercer JE, Karalis T, et al. Effect of restricted feeding on the concentrations of growth hormone (GH), gonadotropins, and prolactin (PRL) in plasma, and on the amounts of messenger ribonucleic acid for GH, gonadotropin subunits, and PRL in the pituitary glands of adult ovariectomized ewes. Endocrinology 1990; 126:1361–7.



28. Frohman LA, Downs TR, Clarke IJ, Thomas GB. Measurement of growth hormone-releasing hormone and somatostatin in hypothalamic-portal plasma of unanesthetized sheep. Spontaneous secretion and response to insulin-induced hypoglycemia. J Clin Invest 1990; 86:17–24.



29. Mueller KM, Themanns M, Friedbichler K, et al. Hepatic growth hormone and glucocorticoid receptor signaling in body growth, steatosis and metabolic liver cancer development. Mol Cell Endocrinol 2012; 361:1–11.



30. Sartin JL, Cummins KA, Kemppainen RJ, et al. Glucagon, insulin, and growth hormone responses to glucose infusion in lactating dairy cows. Am J Physiol 1985; 248:E108–14.


31. Reynaert R, De Paepe M, Marcus S, Peeters G. Influence of serum free fatty acid levels on growth hormone secretion in lactating cows. J Endocrinol 1975; 66:213–24.


32. Katoh K. Developmental and nutritional control of the somatotropic axis in the ruminant. Asian-Australas J Anim Sci 2001; 14:Suppl Issue91–9.
33. Katoh K, Ishiwata H. Changes in intracellular calcium concentration and growth hormone release induced by nutrients in primary cultured anterior pituitary cells of goats. Anim Sci Technol (Japan) 1998; 69:994–1003.

- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





