 |
 |
| Anim Biosci > Volume 30(3); 2017 > Article |
|
Abstract
Objective
Production of alpha-1,3-galactosyltransferase (αGT)-deficient pigs is essential to overcome xenograft rejection in pig-to-human xenotransplantation. However, the production of such pigs requires a great deal of cost, time, and labor. Heterozygous αGT knockout pigs should be bred at least for two generations to ultimately obtain homozygote progenies. The present study was conducted to produce αGT-deficient miniature pigs in much reduced time using mitotic recombination in neonatal ear skin fibroblasts.
Methods
Miniature pig fibroblasts were transfected with αGT gene-targeting vector. Resulting gene-targeted fibroblasts were used for nuclear transfer (NT) to produce heterozygous αGT gene-targeted piglets. Fibroblasts isolated from ear skin biopsies of these piglets were cultured for 6 to 8 passages to induce loss of heterozygosity (LOH) and treated with biotin-conjugated IB4 that binds to galactose-α-1,3-galactose, an epitope produced by αGT. Using magnetic activated cell sorting, cells with monoallelic disruption of αGT were removed. Remaining cells with LOH carrying biallelic disruption of αGT were used for the second round NT to produce homozygous αGT gene-targeted piglets.
Results
Monoallelic mutation of αGT gene was confirmed by polymerase chain reaction in fibroblasts. Using these cells as nuclear donors, three heterozygous αGT gene-targeted piglets were produced by NT. Fibroblasts were collected from ear skin biopsies of these piglets, and homozygosity was induced by LOH. The second round NT using these fibroblasts resulted in production of three homozygous αGT knockout piglets.
Conclusion
The present study demonstrates that the time required for the production of αGT-deficient miniature pigs could be reduced significantly by postnatal skin biopsies and subsequent selection of mitotic recombinants. Such procedure may be beneficial for the production of homozygote knockout animals, especially in species, such as pigs, that require a substantial length of time for breeding.
Despite an increased demand of human organs for transplantation, supply of transplantable human organs is limited. Xenotransplantation may solve this shortage of organs. Among several species, pigs are considered as the most probable species for xenotransplantation because of their compatible size and physiological similarities to humans, breeding characteristics, and availability in specific pathogen-free (SPF) conditions [1,2]. However, porcine organs transplanted to humans may cause severe immune rejection [3]. Hyperacute rejection (HAR) that occurs immediately after transplantation is a major hurdle in pig-to-human transplantation [4,5]. The presence of the epitope galactose-alpha-1,3-galactose (αGal) synthesized by alpha-1,3-galactosyltransferase (αGT) on the surface of porcine cells induces HAR [4,5]. Therefore, the production of αGT-deficient pigs is essential to avoid HAR in pig-to-human xenotransplantation [6].
In an effort to overcome HAR, pigs with targeted disruption of the αGT gene have been produced by somatic cell nuclear transfer (SCNT) [7–10]. Even after the production of animals with heterozygous disruption of the gene, the procedure to produce homozygote pigs requires a great deal of cost, time, and labor. Heterozygote pigs should be either bred at least for two generations to ultimately obtain homozygote pigs.
The procedure to isolate homozygous αGT knockout (KO) cells derived from loss of heterozygosity (LOH) in heterozygous KO cells has been developed [11,12]. Biotin-labeled IB4-lectin and streptavidin-conjugated beads were used to remove cells expressing αGal on their surface. Using this method and subsequent nuclear transfer (NT), homozygous αGT KO pigs were produced from fetal fibroblasts [11]. However, the procedure involves sacrificing heterozygous animals due to collection of fetuses before their term, and it has not been determined whether such procedure could be applied to postnatal cells obtained from heterozygous αGT KO pigs. It would be particularly beneficial for animals with long gestation period to save the time required for breeding for the production of homozygote animals from the verified heterozygotes.
The aim of the current study is to isolate homozygous αGT KO cells from postnatal heterozygous αGT KO skin fibroblasts and ultimately to evaluate the developmental competence of isolated homozygote cells after SCNT.
All procedures in this study were carried out in accordance with the Code of Practice for the Care and Use of Animals for Scientific Purposes and approved by the Institutional Animal Care and Use Committee, Dankook University.
Unless otherwise stated, all chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Pig fetuses at 28 to 39 days of gestation were obtained from Minnesota miniature pigs maintained in SPF conditions at Seoul National University. The head, dorsal spine of the medial section, and tail were removed before collection of embryonic fibroblasts. Briefly, small pieces of remaining tissues were washed in Dulbecco’s phosphate-buffered saline (DPBS; Invitrogen, Carlsbad, CA, USA) and minced with a surgical blade in a 100-mm petridish. Cells were then dissociated from the tissues in 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) (Invitrogen, USA) for 10 min at 39°C. After centrifuging the cell suspension three times at 800×g for 5 min, pellets were resuspended and seeded on 100-mm culture dishes (Corning, Glendale, AZ, USA) and cultured for 6 to 8 days in Dulbecco’s modified Eagle medium (DMEM; Invitrogen, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 1 mM L-glutamine, 100 units/mL penicillin, and 0.5 mg/mL streptomycin in a humidified atmosphere of 5% CO2 in 95% air. After removal of unattached clumps of cells by washing culture plates with DMEM, attached cells were further cultured until confluent and subcultured at intervals of 5 to 7 days by trypsinization until used for transfection and SCNT.
Fetal fibroblasts were cultured to 80% to 90% confluence on 150-mm plates. After harvested with trypsin and washed with DPBS, cells were transfected using Gene Pulser II (Bio-Rad Laboratories, Hercules, CA, USA). Gene targeting vector, as shown in Figure 1, was introduced into fetal fibroblasts. A total of 2×107 cells were suspended in 400 μL of transfection buffer with 10 μg of linearized targeting vector. Electroporation was performed at 250 V, 1,000 mF. Starting from 24 h after transfection, genetically modified cells were selected with culture medium containing 500 μg/mL of G418 (Invitrogen, USA) for 10 to 14 days. After antibiotic selection, G418-resistant colonies were isolated and transferred onto 0.1% gelatin-coated dish for clonal culture. Cells were continuously cultured at 39°C in a humidified atmosphere containing 5% CO2 and 95% air. Genomic DNA was extracted from cells of G418-resistent colonies, and αGT gene targeting events were screened by polymerase chain reaction (PCR) using specific primer sets described previously [2].
The in vitro maturation (IVM) of oocytes and NT were performed as described previously [2]. Briefly, 42 h after the onset of IVM, oocytes were enucleated with a 20-μm (internal diameter) glass pipette by aspiration of the first polar body and the second metaphase plate with a small volume of surrounding cytoplasm in HEPES-buffered TCM-199 supplemented with 0.3% bovine serum albumin (BSA) and 5 μg/mL cytochalasin B. After enucleation, oocytes were stained with 5 μg/mL bisbenzimide (Hoechst 33342) for 5 min and observed under a Nikon TE-300 inverted microscope equipped with epifluorescence. Oocytes containing DNA materials were excluded from the subsequent experiments. Fibroblasts were trypsinized into single cells and transferred into the perivitelline space of enucleated oocytes. The resulting couplets were equilibrated for 1 min in 0.3 M mannitol solution containing 0.5 mM HEPES, 0.05 mM CaCl2, and 0.1 mM MgCl2 in a chamber containing two electrodes. Using a BTX Electro-Cell Manipulator 2001 (Harvard Apparatus, Holliston, MA, USA), couplets were fused with a double DC pulse of 1.5 KV/cm for 45 μs. Following electrical stimulation, reconstructed oocytes were cultured in porcine zygote medium-3 supplemented with 3 mg/mL fatty acid-free BSA and 5 μg/mL cytochalasin B for 3 h in order to suppress extrusion of the second polar body.
On the next day of SCNT, reconstructed embryos were surgically transferred to naturally cycling surrogate sows on the second day of standing estrus. Abdominal ultrasonograpy to test for pregnancy was performed at days 25 to 30 after the embryo transfer. Thereafter, pregnant recipient were examined by ultrasound weekly. At near full-term birth, fetal heartbeat or movement was monitored by Doppler ultrasonography.
Fibroblasts were cultured from ear skin biopsies of the heterozygous αGT KO piglets as previously reported [2]. Selection of homozygous αGT KO cells derived from LOH was performed as described elsewhere [11,12]. Briefly, 5×105 heterozygous αGT KO cells were washed twice with DPBS, resuspended with 1 mL of DPBS containing 4 μg biotin-conjugated IB4-lectin (EY Laboratories, San Mateo, CA, USA) in a 1.5-mL tube, and incubated for 1 h on ice with tapping the tube to prevent precipitation of the cells. After washing cells twice with DPBS by centrifugation, 4 mg of Dynabeads M-280 Streptavidin (Invitrogen, USA) was added to the pellet and the resulting mixture was again incubated as same as the IB4-lectin treatment. Then, the tube was placed on a magnet for 2 to 3 min, and the supernatant was transferred to a new tube on a magnet. This procedure was repeated three times. The final supernatant was cultured in DMEM containing 10% (v/v) FBS in 60-mm culture dish for 10 to 14 days until colonies reached approximately 2 mm in diameter. The culture dish containing fibroblast colonies was washed twice with DPBS and covered with dimethylpolysiloxane (DMPS). Colonies were dissociated by injecting 30 μL of 0.25% trypsin-EDTA on top of each colony underneath DMPS using micropipette. After incubation for 5 min at 39°C, fibroblasts dissociated from each colony were transferred onto 0.1% gelatin-coated 24-well culture dish for clonal culture. Clones of homozygous αGT KO were analyzed by PCR as previously reported [2]. Briefly, genomic DNA was prepared from fibroblasts using DNeasy Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Using Maxime PCR Premix Kit (Intron Biotechnology, Seongnam, Korea) in 20 μL reaction volume, amplification of the target gene was carried out using forward 5′-AGAGGTCGTGACCATAACCAGAT-3′ and reverse 5′-AGCCCATGCTGAACATCAAGTC-3′ primers. Conditions for PCR were as follows: 30 cycles of 15 s at 94°C, 30 s at 65°C, 10 min+20 s increase/cycle at 68°C; and one final cycle of 7 min at 68°C. Amplification products (7.4 and 9.2 kb as shown in Figure 1) were analyzed by 0.4% agarose gel electrophoresis. Colonies that LOH was confirmed by PCR were used for SCNT to produce homozygous αGT KO pigs.
Karyotype analysis of homozygous αGT KO fibroblasts was performed by GenDix, Inc (Seoul, Korea), a commercial provider of karyotyping. Briefly, 200 μL of colecemid (Invitrogen, USA) was added to cells and incubated overnight at 39°C in 5% CO2. Cells were trypsinized, harvested, and centrifuged for 5 min at 400×g. After 10 μL of hypotonic solution (0.075 M KCl) was added to the cell pellet, cells were resuspended, incubated for 20 min at 39°C in 5% CO2, and centrifuged for 5 min at 400×g. Then, hypertonic solution was removed, and 500 μL Carnoy’s fixative (methanol:acetic acid = 3:1) was added and mixed with the cells. After fixative containing fibroblasts was dropped and spread on clean glass slides, the slides were baked 60°C for 30 min and treated with 50% H2O2 for 3 min. Finally, followed by karyotypic analysis following Giemsa staining (GTG banding) was performed using ChIPS-Karyo (Chromosome Image Processing System, GenDix, Seoul, Korea).
To evaluate the expression levels of αGal epitope on cell surface, fibroblasts obtained from wild type, heterozygous αGT KO, and homozygous αGT KO pigs were analyzed by flow cytometry. Cultured cells were harvested, washed with DPBS and resuspended at 7×104/mL. Then cells were labeled with fluorescein isothiocyanate (FITC)-conjugated IB4 and analyzed using BD FACSCalibur Cell Analyzer (BD Biosciences, San Jose, CA, USA).
Southern blot hybridization was performed using DIG High Prime DNA Labeling and Detection Kit 2 (Roche Molecular Systems, Pleasanton, CA, USA). Ten micrograms of genomic DNA were digested with a Bst EII (Takara, Shiga, Japan) overnight at 60°C. As represented in Figure 1, the digestion generates a 6.8 kb fragment in the wild allele and an 8.1 kb fragment in the targeted allele. Samples were separated on a 0.8% agarose gel. Following electrophoresis, restricted genomic DNA was transferred to a nylon membrane (Roche, Switzerland). Then, the membrane was hybridized with a 520 bp DIG-labeled probe in DIG Easy Hyb solution (Roche Molecular Systems, USA) for 16 h at 53°C. After the hybridization, membrane was washed twice in 0.5× SSC with 0.1% sodium dodecyl sulfate at 68°C and exposed to a camera for 10 min.
A total of 2×107 fetal fibroblasts were transfected using αGT gene-targeting vector, and 87 colonies were obtained after antibiotic selection. Among these colonies, two (2.3% efficiency) were identified as heterozygote αGT KO. One of the two was used for subsequent SCNT experiment. A total of 559 cloned embryos were produced by SCNT and transferred to five surrogates. Two pregnancies went to term, leading to delivery of three female piglets by cesarean section (Figure 2A). Genomic DNA was extracted from tail tip of piglets and analyzed by PCR. All of three piglets were confirmed to be heterozygote αGT KO (Figure 2B).
After IB4-Dynabead selection, 22 colonies were isolated from 5×105 cultured fibroblasts obtained from heterozygous αGT KO cloned piglets. Based on PCR analysis, 15 colonies were confirmed to have LOH with biallelic disruption of αGT presumably by mitotic recombination (68.2% LOH efficiency). One of the colonies was chosen for expansion of cells by subculture and subjected to karyotypic analysis. Subcultured homozygous αGT KO fibroblasts demonstrated normal porcine karyotype (Figure 3) and subsequently used for SCNT.
Using the same procedure for the production heterozygous αGT KO pigs, a total of 729 cloned embryos were produced and transferred to four surrogates. Two pregnancies went to term, leading to delivery of four piglets with three viable and one stillborn by cesarean section (Figure 4A). The stillborn piglets had no gross clinical and anatomical abnormalities, and the cause of death was not clear. As represented in Figure 4B and 4C, genomic DNA analyses by PCR and Southern blot showed that all of the piglets were homozygotes with disruption in both alleles of αGT gene.
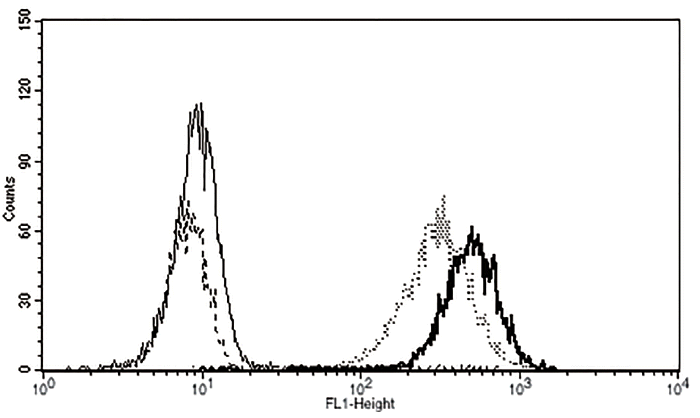
To examine the expression of αGal epitopes on cell membrane, ear skin fibroblasts obtained from wild type, heterozygous, and homozygous αGT KO piglets were reacted with FITC-conjugated IB4 lectin. As shown in Figure 5, unlike wild type and heterozygous αGT KO cells, no fluorescence was observed from homozygous αGT KO fibroblasts, suggesting αGal antigen was not expressed on cell surface of homozygous αGT KO fibroblasts.
In the current study, we demonstrated the procedure that heterozygous αGT KO piglets were produced by conventional gene-targeting technique (Figure 2), and subsequently homozygous αGT KO cells that LOH had occurred were isolated from postnatal heterozygous αGT KO miniature pig ear-skin fibroblasts. Even after extended in vitro culture for induction of LOH, these cells possessed normal karyotype (Figure 3). Ultimately, homozygous αGT KO cells gave rise to the production of homozygous piglets using second round cloning (Figure 4). Although the previous study has shown that homozygous αGT KO could be produced by IB4-Dynabead selection of LOH in heterozygous fetal fibroblasts [11], one drawback of the study is sacrificing heterozygous pigs due to collection of fetuses before the term. Therefore, it needs to be determined whether postnatal fibroblast also could be utilized for the production of homozygous αGT KO pigs by IB4-Dynabead selection. In this way, homozygous KO pigs could be produced in addition to preservation of heterozygous animals
The present study demonstrated that αGT gene could be spontaneously mutated as a result of LOH in postnatal heterozygous αGT KO ear skin fibroblasts. During cell division following genetic mutation, LOH could occur as a consequence of mitotic recombination [13]. Later, homozygous αGT KO pigs were produced from the cells carrying such type of genetic recombination. Flow cytometric analysis using FITC-conjugated IB4 confirmed that the existential level of αGal epitope on cell membrane of homozygous αGT KO piglet was similar to that of unstained cells (Figure 5). The rate of mitotic recombination is dependent on the homology of homologous chromosomes [14,15]. Although we used Minnesota miniature pigs, a nearly inbred miniature pig strain, to isolate homozygous αGT KO fibroblasts, the recovery rate of LOH in the present study was 0.003% (15 PCR-positive homozygous αGT KO colonies from 5×105 heterozygous αGT KO fibroblasts) comparable to the report from Fujimura et al [11]. Even though neonatal fibroblasts were used for the induction of LOH, cloning efficiency of the present study was 0.41% (3 piglets from 729 transferred cloned embryos) which was also comparable to the previous report using fetal fibroblasts [11].
All three live piglets produced by recloning were homozygotes for αGT KO. We took advantage of applying a new technique in colony picking to increase homogeneous cell population. Due to the nature that a disruption of the αGT gene is analyzed by PCR, a contamination of heterozygous αGT KO cells into homozygous αGT KO colony may cause pseudo-negative results from an undesirable amplification of wild type allele of heterozygous αGT KO cells. Therefore, it would be important to strictly isolate individual colonies without such contamination. In the present study, we used the oil-cover method using DMPS to decrease pseudo-negative colonies. Before picking colonies by trypsinization, cells were covered with mineral oil, and the viscosity of oil prevented cells that were detached by enzyme treatment from being scattered. Consequently, it was possible to isolate homogenous colonies without contamination.
The present study demonstrated that postnatal skin biopsy and subsequent selection for LOH could be utilized for the production of homozygous αGT KO piglets. Such procedure could save time and cost for the production of homozygote KO animals by breeding, especially in species that requires substantial length of gestation.
ACKNOWLEDGMENTS
This research was supported by a grant (PJ011375) from the Next-Generation BioGreen 21 Program, Rural Development Administration, a grant (2014034046) supported by the Bio & Medical Technology Development Program, and a grant (2009-0093829) supported by the Priority Research Centers Program through National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning.
Figure 1
A diagram of gene-targeting vector. Arrows, polymerase chain reaction primers to detect homologous recombination; Bar, probe for Southern blot analysis.
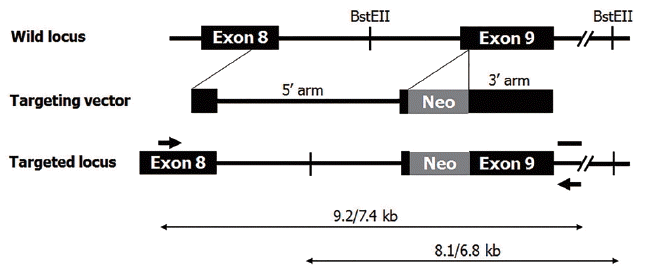
Figure 2
Production of heterozygous αGT KO piglets. A, Heterozygote αGT KO piglets produced by somatic cell nuclear transfer. B, polymerase chain reaction analysis for αGT gene-targeting. αGT, alpha-1,3-galactosyltransferase; KO, knockout.
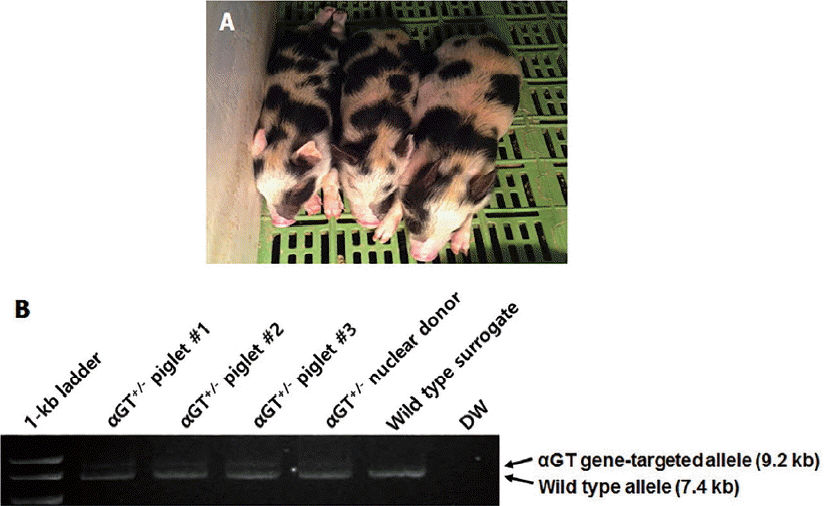
Figure 3
Karyotype of homozygous αGT KO fibroblasts obtained by LOH. Cells contained a normal karyotype of female swine (2n = 38, XX). αGT, alpha-1,3-galactosyltransferase; KO, knockout; LOH, loss of heterozygosity.
Figure 4
Production of homozygous αGT KO piglets. A, Homozygous αGT KO piglets produced by somatic cell nuclear transfer; B, PCR analysis for αGT gene-targeting; C. PCR analysis for αGT gene-targeting. αGT, alpha-1,3-galactosyltransferase; KO, knockout; PCR, polymerase chain reaction.
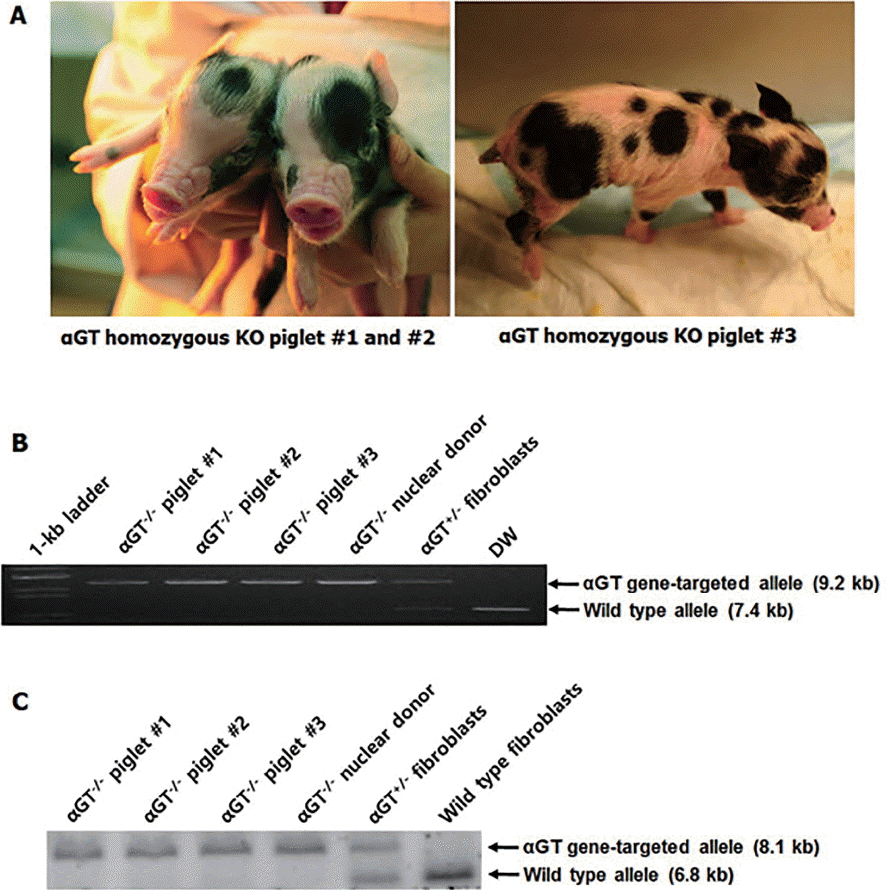
Figure 5
Flow cytometric analysis of fibroblasts from αGT-deficient piglets. This histogram plots a single parameter (FITC intensity, horizontal axis) against the number of cells detected (vertical axis). Thick solid line, wild type cells; Thick dotted line, heterozygous αGT KO cells; Thin dotted line, homozygous αGT KO cells; Thin solid line, unstained cells. αGT, alpha-1,3-galactosyltransferase; KO, knockout; FITC, fluorescein isothiocyanate.
REFERENCES
1. Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol 2007; 7:519–31.


2. Ahn KS, Kim YJ, Kim M, et al. Resurrection of an alpha-1,3-galactosyltransferase gene-targeted miniature pig by recloning using postmortem ear skin fibroblasts. Theriogenology 2011; 75:933–9.


3. Platt JL, Vercellotti GM, Dalmasso AP, Matas AJ, et al. Transplantation of discordant xenografts: a review of progress. Immunol Today 1990; 11:450–6.


4. Joziasse DH, Oriol R. Xenotransplantation: the importance of the Galalpha1,3Gal epitope in hyperacute vascular rejection. Biochim Biophys Acta 1999; 1455:403–18.


5. Galili U. The alpha-Gal epitope (Galalpha1-3Galbeta1-4GlcNAc-R) in xenotransplantation. Biochimie 2001; 83:557–63.


6. Le Bas-Bernardet S, Anegon I, Blancho G. Progress and prospects: genetic engineering in xenotransplantation. Gene Ther 2008; 15:1247–56.


7. Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol 2002; 20:251–5.


8. Lai L, Kolber-Simonds D, Park KW, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002; 295:1089–92.


9. Ramsoondar JJ, Machaty Z, Costa C, et al. Production of alpha 1,3-galactosyltransferase-knockout cloned pigs expressing human alpha 1,2-fucosylosyltransferase. Biol Reprod 2003; 69:437–45.


10. Harrison S, Boquest A, Grupen C, et al. An efficient method for producing alpha(1,3)-galactosyltransferase gene knockout pigs. Cloning Stem Cells 2004; 6:327–31.


11. Fujimura T, Takahagi Y, Shigehisa T, et al. Production of alpha 1,3-galactosyltransferase gene-deficient pigs by somatic cell nuclear transfer: a novel selection method for gal alpha 1,3-Gal antigen-deficient cells. Mol Reprod Dev 2008; 75:1372–8.


12. Waghmare SK, Estrada J, Reyes L, et al. Gene targeting and cloning in pigs using fetal liver derived cells. J Surg Res 2011; 171:e223–9.


13. Hong Y, Cervantes RB, Tichy E, Tischfield JA, Stambrook PJ. Protecting genomic integrity in somatic cells and embryonic stem cells. Mutat Res 2007; 614:48–55.


- TOOLS





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





