 |
 |
| Anim Biosci > Volume 29(6); 2016 > Article |
|
Abstract
Two experiments were conducted assessing the effects of presence or absence of rumen protozoa and dietary nitrate addition on rumen fermentation characteristics and in vitro methane production in Brahman heifers. The first experiment assessed changes in rumen fermentation pattern and in vitro methane production post-refaunation and the second experiment investigated whether addition of nitrate to the incubation would give rise to methane mitigation additional to that contributed by defaunation. Ten Brahman heifers were progressively adapted to a diet containing 4.5% coconut oil distillate for 18 d and then all heifers were defaunated using sodium 1-(2-sulfonatooxyethoxy) dodecane (Empicol). After 15 d, the heifers were given a second dose of Empicol. Fifteen days after the second dosing, all heifers were allocated to defaunated or refaunated groups by stratified randomisation, and the experiment commenced (d 0). On d 0, an oral dose of rumen fluid collected from unrelated faunated cattle was used to inoculate 5 heifers and form a refaunated group so that the effects of re-establishment of protozoa on fermentation characteristics could be investigated. Samples of rumen fluid collected from each animal using oesophageal intubation before feeding on d 0, 7, 14, and 21 were incubated for in vitro methane production. On d 35, 2% nitrate (as NaNO3) was included in in vitro incubations to test for additivity of nitrate and absence of protozoa effects on fermentation and methane production. It was concluded that increasing protozoal numbers were associated with increased methane production in refaunated heifers 7, 14, and 21 d after refaunation. Methane production rate was significantly higher from refaunated heifers than from defaunated heifers 35 d after refaunation. Concentration and proportions of major volatile fatty acids, however, were not affected by protozoal treatments. There is scope for further reducing methane output through combining defaunation and dietary nitrate as the addition of nitrate in the defaunated heifers resulted in 86% reduction in methane production in vitro.
Reviews of the effects of enteric protozoa on digestion and productivity by ruminants have concluded removal of rumen ciliate protozoa reduces enteric methane (CH4) emission by 13% (Newbold et al., 2015) and increases an average daily gain by 11% (Eugène et al., 2004). Finlay et al. (1994) concluded that methanogens existing as endo- and ecto-symbionts with ciliate protozoa contributed 37% of rumen CH4 production and Stumm et al. (1982) identified that 10% to 20% of rumen methanogens were attached on the outside of protozoa. Centrifuging rumen fluid to remove protozoa reduced the methanogen population by 78% (Newbold et al., 1995).
Methane production is positively related to the size of the rumen protozoal population (Morgavi et al., 2010) and the absence of protozoa reduces CH4 production and significantly modifies fermentation characteristics in vitro (Qin et al., 2012). However, Ranilla et al. (2007) reported that there was no correlation between methanogenesis and protozoal biomass per unit of feed degraded in vitro. Further, Bird et al. (2008) showed that defaunation did not change enteric CH4 production 10 to 25 weeks post-treatment. Hegarty et al. (2008) also reported that rumen protozoa did not affect CH4 production by lambs raised without protozoa from birth, or defaunated at weaning. Therefore, the role of protozoa in methanogenesis is unclear.
In contrast, dietary nitrate (NO3) reduces CH4 reliably and predictably (van Zijderveld et al., 2010; 2011). Nitrate reduces total gas production when rumen fluid is incubated in vitro, and it changes the volatile fatty acids (VFA) profile by increasing acetate and reducing propionate and butyrate molar proportions while total VFA concentration is unaffected (Lin et al., 2011).
The objectives of these studies were to describe the fermentation characteristics and CH4 emission changes occurring in the period after refaunation of previously protozoa-free heifers, and assess whether NO3 could further reduce CH4 production from defaunated animals.
All protocols for treatment and care of the cattle were approved by the University of New England Animal Ethics Committee (AEC 13-054). Ten Brahman heifers (8 months of age) with an average liveweight of 274Âą32.8 kg were used. Cattle were adapted to a pre-experimental diet of oaten (70%) and lucerne (30%) chaff with initial inclusion of 1% of coconut oil distillate (COD) which was raised to a final level of 4.5% over 8 d. Cattle were then changed to an experimental diet of for 10 d to eliminate rumen protozoa comprising oaten chaff (70%), lucerne chaff (21%), COD (4.5%) and molasses (4.5%), resulting in 88.1% dry matter (DM) in the mixed ration and 7.9% crude protein and 5% crude fat in the DM. This combined 18 d period of COD dietary treatment reduced the protozoal population from 3.91Ă105 cells/mL to 0.58Ă105 cells/mL of rumen fluid and all cattle were then treated with a chemical to defaunate. After the defaunation treatment, all cattle were given a diet of oaten (70%) and lucerne chaff (30%) which included 10.5% crude protein; 1.3 crude fat; 88.8% DM for the remainder of the study. All cattle had ad libitum access to the ration and water.
After 18 d feeding COD, all feed was withdrawn for a day and cattle were orally dosed with sodium 1-(2-sulfonatooxyethoxy) dodecane (Empicol ESB/70AV, Allright and Wilson Australia Ltd, Melbourne, Australia) administered at 45 g/d in a 10% v/v solution to remove protozoa. Cattle were dosed on three consecutive days and feed was withheld during this treatment protocol, which was described by Bird and Light (2013). Animals required 15 d to fully recover their previous voluntary intake and received the COD diet during this period of time. The three day dosing with Empicol was then repeated commencing 15 d after the first dosing. A further 15 d after the second drenching program, rumen fluid samples were collected for protozoa enumeration and the experiment commenced (d 0).
On d 0 all cattle had recovered their intake and wellbeing, and rumen fluid of the animals was observed to be free of protozoa. Cattle were allocated to defaunated (n = 5) and refaunated groups (n = 5) by stratified randomisation based on liveweight. A single oral dose (500 mL/heifer) of a mixed rumen fluid collected from two cannulated faunated cattle grazing pasture was used to refaunate 5 heifers. The protozoal population in the inoculum (3.42Ă105 cells/mL) consisted of large holotrich (0.13Ă105 cells/mL), small holotrich (0.5Ă105 cells/mL) and small entodiniomorphs (2.79Ă105 cells/mL).
In experiment 1, samples of rumen fluid (40 mL) were collected using oesophageal intubation from defaunated and refaunated heifers before feeding on d 0, 7, 14, and 21. Samples from defaunated heifers were immediately checked under a microscope to confirm that defaunated heifers were protozoa-free. Rumen pH was measured immediately using a portable pH meter (Orion 230 Aplus, Thermo Scientific, Beverly, MA, USA). A 15 mL subsample was placed in wide-neck McCartney bottle acidified with 0.25 mL of 18 M sulphuric acid and stored at â20°C for VFA and ammonia (NH3) analyses. A 4 mL subsample was placed in wide-neck McCartney bottle containing 16 mL of formaldehyde-saline (4% formalin v/v) for protozoa enumeration. Protozoa were counted using a Fuchs-Rosenthal optic counting chamber (0.0625 mm2 and 0.2 mm of depth) using a staining technique adapted from the procedure of Dehority (1984). The protozoa were differentiated into large (>100 Îźm) and small (<100 Îźm) holotrich and entodiniomorph groupings. Another 20 mL of subsample from defaunated and refaunated heifers was used to conduct in vitro incubations for methane measurements.
In experiment 2, samples of rumen fluid (~20 mL) were collected on d 35 using oesophageal intubation from defaunated and refaunated heifers before feeding, with each sample being processed individually and its incubation started immediately after collection. The VFA concentrations were determined by gas chromatography using a Varian CP 3800 Gas Chromatography (Varian Inc. Palo Alto, CA, USA) and NH3 concentration was analysed using a modified Berthelot reaction using a continuous flow analyser (San++, Skalar, Breda, The Netherlands).
In vitro incubations (23 h) were conducted using rumen fluid collected from defaunated and refaunated heifers on d 0, 7, 14, and 21 after refaunation, to assess changes in CH4 production in defaunated and refaunated heifers, while rumen protozoa were re-establishing in refaunated heifers (experiment 1). Samples taken on d 35 were incubated in vitro with the addition of 2% NO3 (as NaNO3) of substrate DM to test for additivity of NO3 and defaunation effects on fermentation and CH4 production (experiment 2). The NaNO3 was dissolved in purified water and added in buffer solution. The composition of incubation buffer was adapted and modified after Soliva and Hess (2007). For all in vitro incubations, 20 mL of rumen fluid from each animal was injected into a Schott bottle (100 mL) which contained 40 mL of buffer solution under a constant flow of anaerobic CO2 in a water bath maintained at 39°C. Mixed rumen fluid and buffer solution (10 mL) was transferred into three 50 mL syringes (Luer lock: Terumo Corporation, Tokyo, Japan) which contained 200Âą20 mg of ground substrate (70% oaten and 30% lucerne chaff). The syringes were sealed by a 3-way tap, pre-warmed to 39°C and then incubated in a shaking water bath at 39°C. After the incubations, gas volume was measured, liquid was drained from the syringes and placed in wide-neck McCartney bottle acidified with 0.25 mL of 18 M sulphuric acid and stored at â20°C for VFA and NH3 analyses. The gas in the syringes were analysed for CH4 concentration using a Varian CP 4900 Gas Chromatography (Varian Inc., USA).
Data were statistically analysed using SAS 9.0 (SAS Inst., Cary, NC, USA). Data from experiment 1 were subject to repeated-measures analysis of variance in PROC MIXED with protozoa, time and protozoaĂtime interaction as fixed factors. Data from experiment 2 were subject to analysis of variance in PROC GLM, factors being protozoa, NO3 and protozoaĂNO3 interaction. Means were analysed using the least squares means (LSMEANS) procedure. A probability of <5% was considered to be statistically significant.
Protozoa were not observed in any rumen fluid samples collected from defaunated heifers during this study. In refaunated heifers, however, the protozoal population reached 3.70Ă105 cells/mL by d 7 and almost doubled by d 21 (7.01Ă105 cells/mL). Small entodiniomorphs were predominant in the total population, accounting for 94%, 82%, and 86% of the total counts at d 7, 14, and 21, respectively (Figure 1). Methane production from refaunated heifers was positively correlated with protozoal numbers although CH4 production tended to stabilise after d 14 (Figure 2).
The rumen fluid pH was higher (p = 0.02) in refaunated heifers, but increased (p<0.001) from d 0 to d 21 in both defaunated and refaunated heifers, showing effects of protozoal treatments and time (Table 1). Ammonia concentrations increased steadily up to d 7 in both defaunated and refaunated heifers, but refaunated heifers had higher NH3 concentrations than did defaunated heifers (p<0.05). Neither VFA concentration, nor molar proportions of acetate, propionate and butyrate in total VFA, or acetate to propionate ratio were affected by protozoal treatment, but all except butyrate proportion increased over time.
There was an increase in total gas production in vitro by rumen fluid collected from both defaunated and refaunated heifers from d 0 to 14 with no significant further increase to d 21. There was a tendency towards a lower CH4 production from rumen fluid of defaunated heifers than from refaunated heifers over time (p = 0.07). No significant interaction between protozoal treatment and time was observed (p>0.05).
The pH after incubation was increased by the presence of protozoa and by NO3 (Table 2). Ammonia concentration was also increased by protozoal treatments and by NO3 (p<0.05). The presence of protozoa had little effect on VFA, with total VFA concentration tending to be lower in rumen fluid from defaunated than refaunated heifers, but VFA proportions were unaffected. VFA concentration was significantly reduced by NO3 and a significant reduction in butyrate percentage also occurred.
Methane production was reduced by both defaunation and by NO3, and there was a significant interaction between defaunation and NO3 such that mitigation resulting from NO3 and defaunation was greater than the mitigation resulting from either alone (p<0.05). Methane production from defaunated heifers was lower than from refaunated ones (18.59 vs 22.11 mL/g DM). While NO3 reduced the CH4 production in refaunated heifers (12.73 vs 22.11 mL/g DM), the combined effects of defaunation and dietary NO3 on CH4 mitigation (19.11 mL) was greater than the sum of effects of defaunation (3.52 mL) and NO3 (9.38 mL), implying the combined treatments were more than additive in their mitigation potential. Total gas production was not affected by protozoal treatments (p>0.05), but was reduced in incubations containing NO3 (p<0.05).
The objectives of this study were to describe the changes in CH4 production and rumen fermentation characteristics associated with the reintroduction of protozoa into previously protozoa-free heifers and also assess whether CH4 mitigation arising from NO3 would be additive to that caused by the absence of protozoa. The protozoal population in previously defaunated heifers was established by d 7 and reached 7.01Ă105 cells/mL by d 21 comparable with that found by Morgavi et al. (2008) in sheep. These authors demonstrated that total protozoal population reached their peak at 12Ă105 cells/mL at 25 to 30 d after inoculation and then stabilised at 7.6Ă105 cells/mL from d 60. During the refaunation period there was a substantial increase in CH4 production rate; this result was in accordance with the positive correlation between protozoa and CH4 production found by Morgavi et al. (2010) and the fact methanogens that are normally attached to protozoa (Newbold et al., 1995) are responsible for 37% of rumen CH4 emission (Finlay et al., 1994). The present study showed that rumen fluid from previously defaunated heifers tended to have lower CH4 production in vitro than samples from refaunated heifers 7, 14, and 21 d after refaunation. This effect may not be exclusively a direct consequence of protozoa but also an indirect consequence of differences in bacterial and fungal populations in the presence of protozoa (Eugène et al., 2004) and in some cases, an increase in activity of H2 producers (Morgavi et al., 2012). Such compensatory changes in microbial populations after defaunation leading to an unchanged VFA pattern may explain why the absence of protozoa has caused no significant changes in CH4 emission in defaunated animals as observed from some previous studies (Bird et al., 2008; Hegarty et al., 2008; Morgavi et al., 2012).
Effects of protozoa on rumen NH3 concentrations are generally more consistent than effects on VFA concentration with the concentration of NH3 lower in defaunated ruminants compared to faunated or refaunated ones in this and previous studies (Jouany et al., 1988; Eugène et al., 2004; Santra et al., 2007; Morgavi et al., 2012; Newbold et al., 2015). Defaunation has sometimes increased total VFA concentration in defaunated sheep (Santra et al., 2007) and weaner lambs (Santra and Karim, 2002), but Hegarty et al. (2008) found total VFA was lower and the proportion of propionate was reduced in the protozoa-free lambs born from defaunated ewes. These authors suggested that effects of defaunation on reducing CH4 production may be dependent upon fermentation shifting to a more propionate rich pattern in defaunated animals. This is consistent with defaunation normally increasing the proportion of propionate and decreasing the proportion of butyrate while concomitantly reducing methane output (Eugène et al., 2004; Morgavi et al., 2012). No differences between defaunated and refaunated heifers in concentration and proportions of VFA were observed in these studies but the absence of rumen protozoa still reduced CH4 production (experiment 2), indicating that protozoal effects on methanogenesis are not just a consequence of increased partitioning of H2 into propionate synthesis.
Importantly, the successive in vitro studies showed that despite defaunation being completed 15 d before d 0; the rumen of defaunated heifers was not metabolically stable, with pH, total VFA, NH3 and CH4 production changing out to d 21 in the experiment 1. Little is known about rumen ecological stabilisation after defaunation, it was presumable in these studies that rumen ecology was stable within 50 d after defaunation and therefore was stable when the combined effects of NO3 and defaunation were assessed in experiment 2.
Dietary NO3 has been shown to offer a reliable and predictable strategy to mitigate CH4 production from ruminants in both in vitro and in vivo studies. A review by Leng and Preston (2010) concluded that the use of NO3 as a hydrogen sink could reduce CH4 production from 16% to 50%, depending on diets and the inclusion rate of NO3. This is because approximately 2 moles of hydrogen will be needed to convert NO3 to nitrite and 6 moles hydrogen will be removed in order to reduce nitrite to NH3 (Allison and Reddy, 1984). The result from the experiment 2 showed that CH4 production was significantly lowered by addition of NO3 in refaunated heifers 35 d after refaunation, confirming the potential for role of dietary NO3 as a strategy to mitigate CH4 emission (Guo et al., 2009; Nolan et al., 2010; van Zijderveld et al., 2010; 2011). In addition, NO3 reduced total gas production, total VFA concentrations and the proportion of butyrate in vitro in line with findings of Lin et al. (2011). The present study also indicated that the combined effects of protozoal treatment and dietary NO3 led to more than additive reduction in CH4 production (19.11 mL) compared with the sum of the protozoal effect (3.52 mL) and the dietary NO3 effect (9.38 mL).
Methane production was positively correlated with protozoal numbers in rumen fluid in the period following refaunation of defaunated heifers with protozoa. The absence of protozoa reduced CH4 production by 16% compared with refaunated heifers, dietary NO3 reduced CH4 production by 42% and the combined effects of NO3 and defaunation reduced CH4 production by 86%. Future research is needed to confirm these suggestions and gain better understandings the changes in gut fermentation, adaptation of methanogens and increased activity of some rumen microbes after defaunation and refaunation. In vivo trials need to be undertaken to gain a better understanding of the combined effects of defaunation and dietary NO3 on CH4 production in ruminants.
ACKNOWLEDGMENTS
This research was funded by Meat and Livestock Australia and the Australian Government Department of Agriculture, Fisheries and Forestry Carbon Farming Futures, Filling the Research Gap Program. The authors are grateful for the assistance of Dr Simon Bird and Professor John Nolan in discussions and guidance. All helps from Mr Graeme Bremner, Andrew Blakely and Mrs Jennies Hegarty with technical supports are acknowledged.
Figure 1
Small holotrich (âĄ), large holotrich (â ) and small entodiniomorphs (
 ) from refaunated heifers 7, 14 and 21 d after refaunation.
) from refaunated heifers 7, 14 and 21 d after refaunation.
 ) from refaunated heifers 7, 14 and 21 d after refaunation.
) from refaunated heifers 7, 14 and 21 d after refaunation.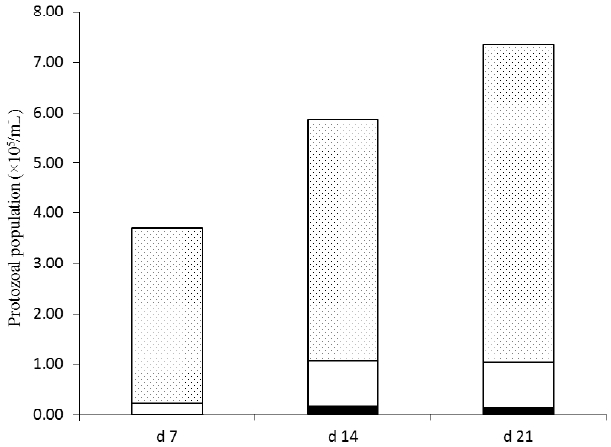
Figure 2
Methane production (âĄ) and protozoal population (â ) in rumen fluid from refaunated heifers 0, 7, 14, and 21 d after refaunation using a mixed rumen fluid inoculum. Error bars indicate standard error of the mean.

Table 1
The pH, ammonia concentration and concentration and molar proportions of major volatile fatty acids (VFA) in rumen fluid, and changes in gas and methane production in-vitro after refaunation
| Item1 | Treatment | SEM | p-values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Defaunated | Refaunated | Trt | Time | TrtĂtime | ||||||||
|
|
|
|||||||||||
| Day 0 | Day 7 | Day 14 | Day 21 | Day 0 | Day 7 | Day 14 | Day 21 | |||||
|
|
||||||||||||
| pH | 6.41 | 6.46 | 6.87 | 6.83 | 6.62 | 6.69 | 6.86 | 6.91 | 0.10 | 0.02 | <0.001 | 0.34 |
| Ammonia (mg/L) | 32.68 | 30.76 | 59.04 | 62.92 | 36.88 | 69.52 | 86.24 | 117.00 | 9.56 | <0.01 | <0.001 | 0.08 |
| Total VFA(mM/L) | 64.43 | 59.67 | 50.92 | 57.95 | 59.46 | 63.43 | 63.03 | 58.16 | 8.05 | 0.63 | 0.18 | 0.39 |
| VFA molar proportion (%) | ||||||||||||
| âAcetate (%) | 71.06 | 74.55 | 75.32 | 79.01 | 73.67 | 73.49 | 73.39 | 76.74 | 1.76 | 0.59 | 0.04 | 0.51 |
| âPropionate (%) | 19.15 | 16.61 | 15.05 | 14.46 | 17.75 | 15.66 | 14.52 | 12.30 | 1.40 | 0.12 | 0.02 | 0.95 |
| âButyrate (%) | 8.38 | 7.05 | 6.54 | 6.39 | 6.77 | 8.03 | 8.44 | 7.39 | 0.65 | 0.37 | 0.60 | 0.03 |
| âAcetate/propionate | 4.07 | 4.65 | 5.08 | 5.57 | 4.58 | 4.77 | 5.07 | 6.29 | 0.51 | 0.26 | 0.44 | 0.90 |
| Total gas2 (mL/g DM) | 102.33 | 128.67 | 144.07 | 157.00 | 103.67 | 135.67 | 152.00 | 149.33 | 4.71 | 0.55 | <0.001 | 0.34 |
| CH4 (mL/g DM) | 6.44 | 13.60 | 16.86 | 20.66 | 6.99 | 16.76 | 21.68 | 21.47 | 1.29 | 0.07 | <0.001 | 0.19 |
Table 2
The pH, ammonia concentration, volatile fatty acid (VFA) concentration and molar proportions and methane production as influenced by the presence or absence of protozoa (F, fauna) or nitrate (NO3) addition for incubations of rumen fluid in-vitro
REFERENCES
Allison MJ, Reddy CA. 1984. Adaptations of gastrointestinal bacteria in response to changes in dietary oxalate and nitrate. Third International Symposium on Microbial Ecology. Klug MJ, Reddy CA, editorsWashington, DC, USA: Am. Soc. Microbiol; p. 248â256.
Bird SH, Hegarty RS, Woodgate R. 2008. Persistence of defaunation effects on digestion and methane production in ewes. Aust J Exp Agric 48:152â155.

Bird SH, Light MN. 2013. A protocol for the removal of protozoa (defaunation) from the rumen of cattle. Proceeding of the Recent Advances in Animal Nutrition in Australia. Cronje P, editorUniversity of New England Publishing Unit; Armidale, Australia: p. 1â2.
Dehority BA. 1984. Evaluation of subsampling and fixation procedure used for counting rumen protozoa. Appl Environ Microbiol 48:182â185.



Eugène M, Archimède H, Sauvant D. 2004. Quantitative meta-analysis on the effects of defaunation of the rumen on growth, intake and digestion in ruminants. Livest Prod Sci 85:81â97.

Finlay BJ, Esteban G, Clarke KJ, Williams AG, Embley TM, Hirt RP. 1994. Some rumen ciliates have endosymbiotic methanogens. FEMS Microbiol Lett 117:157â161.


Guo WS, Schaefer DM, Guo XX, Ren LP, Meng QX. 2009. Use of nitrate-nitrogen as a sole dietary nitrogen source to inhibit ruminal methanogenesis and to improve microbial nitrogen synthesis in vitro. Asian Australas J Anim Sci 22:542â549.

Hegarty RS, Bird SH, Vanselow BA, Woodgate R. 2008. Effects of the absence of protozoa from birth or from weaning on the growth and methane production of lambs. Br J Nutr 100:1220â1227.


Jouany JP, Demeyer DI, Grain J. 1988. Effect of defaunating the rumen. Anim Feed Sci Technol 21:229â265.

Leng RA, Preston TR. 2010. Further considerations of the potential of nitrate as a high affinity electron acceptor to lower enteric methane production in ruminants. Livest Res Rural Dev 22:221
Lin M, Schaefer DM, Guo WS, Ren LP, Meng QX. 2011. Comparisons of in vitro nitrate reduction, methanogenesis, and fermentation acid profile among rumen bacterial, protozoal and fungal fractions. Asian Australas J Anim Sci 24:471â478.

Morgavi DP, Forano E, Martin C, Newbold CJ. 2010. Microbial ecosystem and methanogenesis in ruminants. Animal 4:1024â1036.


Morgavi DP, Jouany JP, Martin C. 2008. Changes in methane emission and rumen fermentation parameters induced by refaunation in sheep. Aust J Exp Agric 48:69â72.

Morgavi DP, Martin C, Jouany JP, Ranilla M. 2012. Rumen protozoa and methanogenesis: not a simple cause-effect relationship. Br J Nutr 107:388â397.


Newbold CJ, Lassalas B, Jouany JP. 1995. The importance of methanogensis associated with ciliate protozoa in ruminal methane production in vitro. Lett Appl Microbiol 21:230â234.


Newbold CJ, de la Fuente G, Belanche A, Ramos-Morales E, McEwan N. 2015. The role of ciliate protozoa in the rumen. Front Microbiol 6:Article 1313



Nolan JV, Hegarty RS, Hegarty J, Godwin IR, Woodgate R. 2010. Effects of dietary nitrate on fermentation, methane production and digesta kinetics in sheep. Anim Prod Sci 50:801â806.

Qin WZ, Li CY, Kim JK, Ju JG, Song MK. 2012. Effects of defaunation on fermentation characteristics and methane production by rumen microbes in vitro when incubated with starchy feed sources. Asian Australas J Anim Sci 25:1381â1388.



Ranilla MJ, Jouany JP, Morgavi DP. 2007. Methane production and substrate degradation by rumen microbial communities containing single protozoal species in vitro. Lett Appl Microbiol 45:675â680.


Santra A, Karim SA. 2002. Influence of ciliate protozoa on biochemical changes and hydrolytic enzyme profile in the rumen ecosystem. J Appl Microbiol 92:801â811.


Santra A, Karim SA, Chaturvedi OH. 2007. Rumen enzyme profile and fermentation characteristics in sheep as affected by treatment with sodium lauryl sulfate as defaunating agent and presence of ciliate protozoa. Small Rumin Res 67:126â137.

Soliva CR, Hess HD. 2007. Measuring methane emission of ruminants by in vitro and in vivo. Measuring Methane Production from Ruminants. Harinder PSM, Philip EV, editorsSpringer; Netherlands: p. 15â31.

Stumm CK, Gijzen HJ, Vogels GD. 1982. Association of methanogenic bacteria with ovine rumen ciliates. Br J Nutr 47:95â99.


- TOOLS
-
METRICS

- Related articles
-
Effects of zinc-bearing palygorskite on rumen fermentation in vitro2019 January;32(1)





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




