Gut Health of Pigs: Challenge Models and Response Criteria with a Critical Analysis of the Effectiveness of Selected Feed Additives — A Review
Article information
Abstract
The gut is the largest organ that helps with the immune function. Gut health, especially in young pigs has a significant benefit to health and performance. In an attempt to maintain and enhance intestinal health in pigs and improve productivity in the absence of in-feed antibiotics, researchers have evaluated a wide range of feed additives. Some of these additives such as zinc oxide, copper sulphate, egg yolk antibodies, mannan-oligosaccharides and spray dried porcine plasma and their effectiveness are discussed in this review. One approach to evaluate the effectiveness of these additives in vivo is to use an appropriate disease challenge model. Over the years, researchers have used a number of challenge models which include the use of specific strains of enterotoxigenic Escherichia coli, bacteria lipopolysaccharide challenge, oral challenge with Salmonella enteric serotype Typhimurium, sanitation challenge, and Lawsonia intercellularis challenge. These challenge models together with the criteria used to evaluate the responses of the animals to them are also discussed in this review.
INTRODUCTION
Gut health is a term increasingly used in the medical literature and by the food industry. It covers multiple positive aspects of the gastrointestinal tract (GIT), such as the effective digestion and absorption of food, the absence of gastrointestinal illness, normal and stable intestinal microbiota, effective immune status and a state of well-being (Bischoff, 2011). The GIT of a pig is a complex environment. Particularly in newborns and around the time of weaning, the pigs’ gut rapidly changes in size, has high protein turnover rates, undergoes rapid changes in microbiota, and quickly alters its digestive and immune functions (Pluske et al., 1997).
The correct functional development of the GIT is of special importance during the weaning phase of reared piglets (Domeneghini et al., 2006). The mucosal epithelium of the small intestine is reputed anatomically and functionally immature in neonatal pigs, a feature that appears to be exacerbated at weaning, when a colonization of the gut occurs by new microorganisms entering the alimentary canal with solid feed. This frequently exposes piglets to diarrhoeic syndromes and other intestinal disturbances (Domeneghini et al., 2006). In the lifespan of the pig, the neonatal and weaning phases represent critical periods for both the correct development of the gut and the growth of the young animal (Pluske et al., 1997; Lay et al., 2001). The correct and timely functional development of the GIT, in which it should be able to sustain growing digestive, absorptive and immune functions, is of particular importance for these adaptable processes correctly occurring (Domeneghini et al., 2006). The GIT serves a key functional role in the growth of the young piglet, even though it represents a relatively small fraction of its body weight (BW), in that it is approximately 2% of BW at birth and increases nearly threefold, to more than 6%, two weeks after weaning (Shields et al., 1983; Mitchell et al., 2001).
To improve productivity of pigs, researchers have evaluated a wide range of feed additives (such as immunoglobulin, omega 3 fatty acids, yeast derived B glucans, organic and inorganic acids, high levels of zinc oxide, essential oils, herbs and spices, some types of prebiotics, bacteriophages and anti-microbial peptides, probiotics, glutamine, threonine, cysteine, and nucleotides) as substitute products for antibiotics. The role of these additives and their potential use in managing gut health and function in newly weaned pigs has been reviewed extensively elsewhere (de Lange et al., 2010; Heo et al., 2013; Thacker, 2013; Zeng et al., 2015).
An approach to evaluate the efficacy of various additives as replacements for antibiotics in vivo is to use an appropriate disease challenge model. Over the years, researchers have used a number of in vivo challenge models some of which were discussed in this review.
DISEASE CHALLENGE MODELS
Post-weaning diarrhea using specific pathogenic strains of enterotoxigenic Escherichia coli
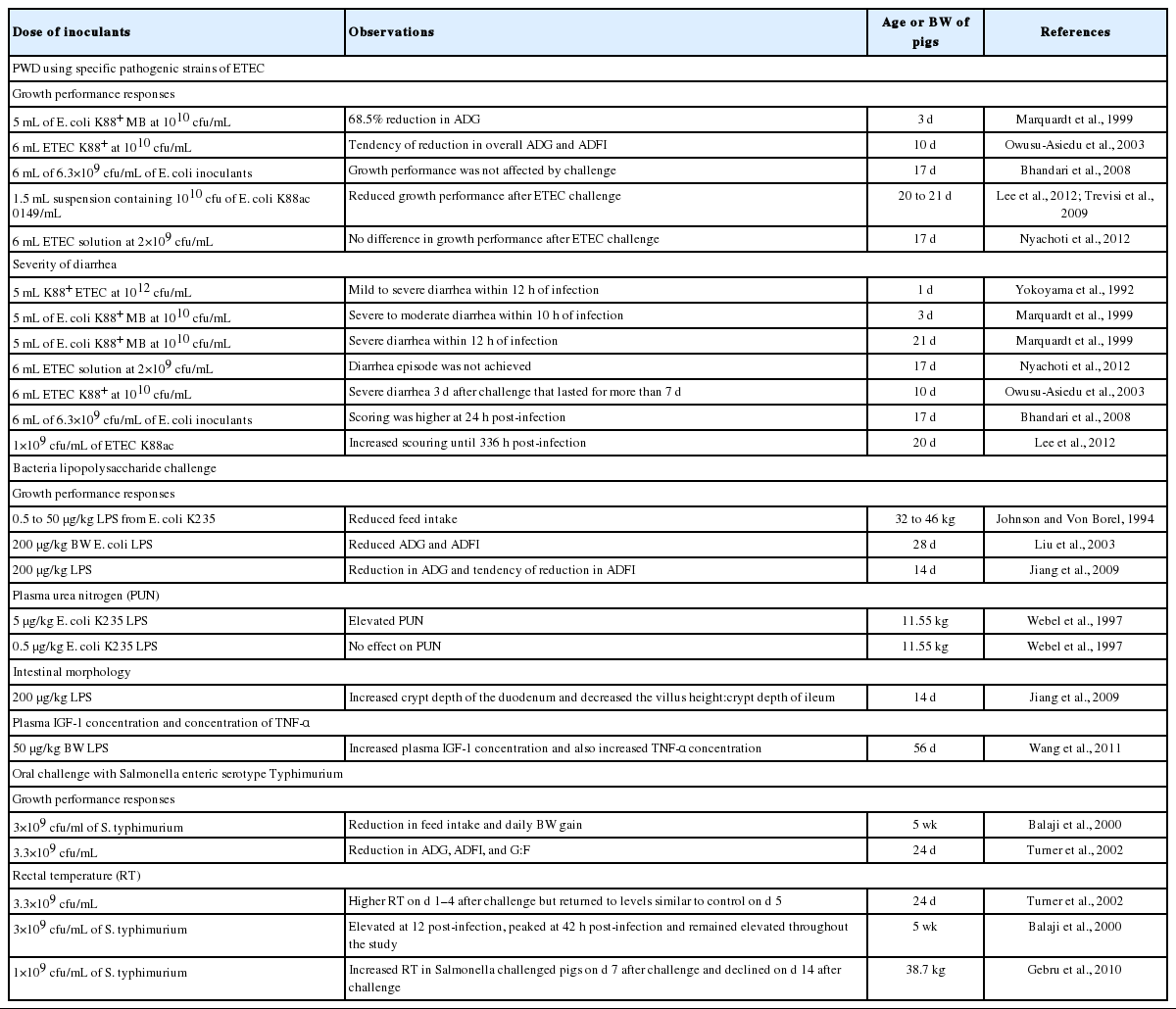
Enterotoxigenic Escherichia coli (ETEC) is the main causative agent of diarrhea in weaned pigs. It attaches to and effaces the intestinal mucosa, thus leading to malabsorption of large molecules as a result of a compromised barrier function. The ETEC diarrhea is the most common enteric disease in piglets, accounting for 50% of the piglets that die annually worldwide (Gyles, 1994). Investigations have indicated that colonization of the small intestine of the piglet by ETEC adhering to the epithelium accounts for most gastrointestinal disorders (Yolken et al., 1988) (Table 1). The fimbrial K88, K99, and 987P antigens of porcine ETEC that are associated with intestinal colonization have been extensively investigated with respect to their genetic background, protein chemistry, and immunological properties (Fusco et al., 1978).
Enterotoxigenic Escherichia coli (E. coli) K88 is the pathogen most frequently isolated in piglets and associated with colibacillosis. Studies (Owusu-Asiedu et al., 2002; Owusu-Asiedu et al., 2003; Bhandari et al., 2008) have demonstrated the suitability of ETEC K88 challenge model in evaluating the role of feed additives in nursery pig diets. Oral challenge with ETEC has been used widely as model for post-weaning diarrhea (PWD) (Wellock et al., 2009) and has often been used in pig feeding trials to assess the ability of a diet to reduce the infection or its consequences (Bosi et al., 2004a, b). Dietary phytogenics could be used to replace antibiotic growth promoters (AGP) in the diets of weaning pigs for significantly improving the average daily gain (ADG) without any negative effects on growth performance under challenge with E.coli K88 compared with AGP and organic acids (Mohana Devi et al., 2015).
Response criteria
In this challenge model, a number of response criteria are usually used. The most common among them are growth performance, fecal consistency score, plasma urea nitrogen (PUN), fecal and tissue bacteria number, intestinal morphology (villus height [VH] and crypt depth [CD]), serum concentrations of haptoglobin, tumor necrosis factor (TNF-α), interleukin 6 (IL-6), and interferon-gamma (INF-γ), number of intestinal adherent bacteria, digesta pH, rectal temperature (RT) and mortality. Some of these response criteria are discussed below.
Growth performance
Average daily feed intake (ADFI), ADG, and feed conversion efficiency (G:F) have been used as response criteria during ETEC challenge. Marquardt et al. (1999) orally challenged pigs with 5 mL of E. coli K88+ at a dose of 1010 colony-forming unit (cfu)/mL and reported that pigs lost weight within 48 h post-challenge. At 72 h after ETEC challenge, Marquardt et al. (1999) reported 68.5% reduction in ADG. Using the same ETEC strain, Owusu-Asiedu et al. (2003) reported no effect but a tendency of reduction in ADFI and ADG upon challenge. Bhandari et al. (2008) and Nyachoti et al. (2012) similarly reported that growth performance was not affected by ETEC challenge. However, reduction in growth performance was reported by a number of researchers (Trevisi et al., 2009; Song et al., 2011; Lee et al., 2012).
The reason why a reduction in growth performance was not achieved in all cases as expected is worthy of investigation. Factors to be considered are: The species of pigs used in the different experiments, age of pigs at the time of challenge, variation in the volume and dosage of inoculants used, buffer type and method of delivery of the inoculants into the oral cavity of pigs, health status of pigs prior to ETEC challenge, duration of the experiment and duration of acclimatization prior to challenge. For example, Trevisi et al. (2009) used 1.5 mL of inoculant as opposed to the 5 mL used by Marquardt et al. (1999) and the 6 mL used by Nyachoti et al. (2012). Also, Bhandari et al. (2008) and Marquardt et al. (1999) used a concentration of 6.3×1010 cfu/mL and 5×1010 cfu/mL of ETEC inoculants, respectively, as opposed to the 1×1010 used by other researchers (Owusu-Asiedu et al., 2003).
Fecal consistency score
This has been used by many researchers to quantify the severity of diarrhea. The scoring method commonly used is the 0 to 3 scoring system; where 0 = normal feces, 1 = soft feces, 2 = mild diarrhea, and 3 = severe diarrhea. Yokoyama et al. (1992) reported that all piglets developed mild to severe diarrhea within 12 h of ETEC infection. Similarly, Owusu-Asiedu et al. (2003) reported that 3 d after oral ETEC challenge, piglets had severe diarrhea with scour score of 2.8 that lasted for more than 7 d. Nyachoti et al. (2012) reported that fecal consistency score reduced from 1.07 at 6 h post-infection to 0.94 at 120 h post-challenge. Song et al. (2011) reported that E. coli challenge did increase diarrhea scores and frequency. Lee et al. (2012) reported that ETEC challenge increased scouring until 336 h post-infection. Kahindi (2014) after inoculating pigs with 6 and 15 mL of ciprofloxacin-resistant ETEC K88+ (5×109 cfu/mL), reported that pigs had normal feces pre-challenge, but fecal score slightly changed in the post-challenge period due to increased incidence of diarrhea.
Plasma urea nitrogen
Nitrogen metabolism in pigs has been evaluated as a rapid response indicator of amino acid concentrations in diets. PUN has been used as an indicator for amino acids breakdown as a result of less than optimal systemic amino acids supply for protein synthesis (Coma et al., 1996) and of muscle protein breakdown to release amino acids for synthesizing acute phase proteins in the liver as a response to immune system activation (Wannemacher, 1977). Owusu-Asiedu et al. (2003) reported that PUN levels were higher upon ETEC challenge. The results of Kiarie et al. (2009) also indicated that pigs had elevated PUN at 24 h post-challenge. However, Bhandari et al. (2008) reported contrarily that PUN reduced from 6.23 to 5.53 mmol/L after 7 d of infection with ETEC. The higher PUN concentration in the challenged pigs as reported by Owusu-Asiedu et al. (2003) and Kiarie et al. (2009) could either be an indication of increased muscle protein breakdown to release amino acids for synthesizing acute phase proteins in the liver and/or to serve as an energy source because of lower feed intake in this group of pigs.
Intestinal morphology
The morphology of the intestinal mucosa is an important indicator that reflects the development of the digestive tract and the response of intestine to certain feed substances (Boguslawka-Tryk et al., 2012). It is commonly believed that an increased VH and a decreased crypts depth is positively correlated to the digestive and absorptive functions in the GIT of animals, accounting for an enlarged absorptive area and a reduced tissue turnover rate (Xu et al., 2003; Munyaka et al., 2012; Shang, 2014). Opapeju et al. (2009) reported no difference in VH and CD after 7 d of ETEC challenge. No difference in VH:CD was observed by Trevisi et al. (2009) after 7 d of ETEC challenge as well. However, shorter villi were observed in the duodenum by Owusu-Asiedu et al. (2003) after ETEC challenge. CDs were similar and were not influenced by ETEC challenge (Owusu-Asiedu et al., 2003).
Serum concentrations of haptoglobin, tumor necrosis factor α, interleukin-6, and interferon-gamma
These pro-inflammatory cytokines play a critical role in normal host resistance to infection, serving as immunomodulators and as mediators of inflammatory responses. They are primarily produced by activated macrophages through numerous signals, such as mitogenic or antigenic stimulation, lipopolysaccharide (LPS), calcium ionophores, cytokines and viruses. Serum IL-6, IL-1β, and IL-10 increased with time post-challenge (Kiarie et al., 2009). Similarly, Nyachoti et al. (2012) reported that TNF-α increased from 111.4 to 162.1 g/mL upon ETEC challenge. Also, Lee et al. (2012) reported increased concentration of serum haptoglobin, TNF-α, IL-6, and INF-γ compared with control. Recently, Kahindi (2014) reported an increase in TNF-α concentration after 6 h inoculation with 6 and 15 mL of ciprofloxacin-resistant ETEC K88+ (5×109 cfu/mL).
Lipopolysaccharide challenge
Lipopolysaccharide is a molecule present on the outer surface of all gram-negative bacteria which is widely used as an immune stimulant (Chapman et al., 2005). It is a potent inflammatory mediator and has been used to model bacterial infection experimentally in farm animals (Yang et al., 2008). Many aspects of inflammation caused by infection with gram-negative bacteria can be mimicked by administration of LPS (Shini et al., 2008 as cited by Munyaka, 2012). Lipopolysaccharide challenge is a well-documented model for inducing symptoms of acute bacterial infection and immunological stress in laboratory animals. The biological effects mediated by LPS are attributed to a cascade of cytokine synthesis and release by stimulated macrophages (Dinarello, 1984) and a subsequent increase in prostaglandin synthesis (Hashimoto et al., 1988). It is well documented that an immunological challenge can result in a series of physiological changes including increased body temperature, depressed feed intake, changes in plasma acute phase protein concentration, activation of the immune system, activation of the hypothalamic-pituitary-adrenal axis, and inhibition of the somatotropic axis (Johnson, 1997). Consequently the immunological challenge results in the poor growth of livestock and may increase economic loss for animal producers (Liu et al., 2003). Zhang et al. (2008) reported a growth improving effect of β-glucan at 50 and 75 mg/kg in the diet in broilers.
One emerging view is that these changes are attributed to the release of pro-inflammatory cytokines (Johnson, 1997). The effects of LPS are due to its ability to stimulate macrophages to synthesize and secrete pro-inflammatory cytokines (Johnson and von Borrel, 1994). Whether LPS is indicative of on-farm conditions is a subject of much debate; however, it is obvious that its administration activates several immune defences, which allows one to better understand the physiology of infection and inflammation due to an immunological challenge (Liu et al., 2003). According to Mohsen and Kim (2014), supplementation of stevia in growing pigs improves blood immunity and alleviates the inflammatory responses in LPS challenge.
Response criteria
In pigs, LPS which is the primary toxic component of endotoxin induces symptoms of acute bacterial infection, including anorexia, hypersomnia, and fever. The most common response criteria include growth performance, PUN, concentrations of TNF-α, IL-1, and IL-6, plasma cortisol, insulin-like growth factor 1 (IGF-1), and growth hormone (GH) concentrations, and Intestinal morphology which are discussed below.
Growth performance
Johnson and Von Borel (1994) reported that growing pigs injected with 0.5 or 50 μg/kg LPS from E. coli serotype K-235 had lower feed intake compared with control after 2 h. At 4 to 8 h, only the pigs receiving the highest dose (50 μg/kg) of LPS experienced a reduction in feed intake and feed motivated behaviour whereas pigs receiving 0.5 μg/kg demonstrated a compensatory increase in feed intake suggesting that although LPS is a potent inhibitor of feed-motivated behaviour, its effects are short-lived. Following the same model, Liu et al. (2003) administered E. coli LPS serotype 055:B5 as an inflammatory agent. During the first challenge period (d 14 to 21), LPS challenge reduced ADG and ADFI compared with the saline-treated pigs. During the second challenge period (d 21 to 28), pigs challenged with LPS had lower ADG than saline-treated pigs but ADFI was not affected. This indicated that the response of pigs to the first LPS was stronger than the response to the second injection. In another study by Jiang et al. (2009), 200 μg/kg LPS was injected intramuscularly on d 7 and 14 after blood sampling. During the challenge period (d 7 to 21), inflammatory challenge with LPS reduced ADG and tended to reduce ADFI compared with that of phosphate-buffered saline treated piglets. Inclusion of 0.1% β-glucan and 0.1% kefir, either individually or combined, would improve growth performance and benefit meat quality in broiler chickens (Cho et al., 2013)
Plasma urea nitrogen
PUN is an indicator of protein catabolism in feed-deprived animals. Increased protein degradation is a typical response in early feed-deprived conditions because amino acids are needed as gluconeogenic substrates in the liver (Webel et al., 1997). Webel et al. (1997) reported that PUN levels were elevated two- and threefold at 8 and 12 h respectively, in pigs injected with 5 μg/kg LPS E. coli serotype K-235. Whereas administration of 0.5 μg/kg LPS had no effect on PUN levels. There was a trend for increased PUN levels at 24 h after injection that could be explained by proteolysis typical of an extended period without feed.
Concentrations of TNF-α, IL-1, and IL-6
Intraperitoneal injection of LPS to pigs increased plasma levels TNF-α and IL-6 (Webel et al., 1997). It also increased plasma level of IL-1 and skeletal muscle protein degradation in rodents (Jepson et al., 1986; Fong et al., 1989; Goodman, 1991). Serum TNF-α reached peak concentrations after 1 h LPS administration and returned to near baseline levels 3.5 h after challenge (Frank et al., 2003). Also, LPS challenge (50 μ/kg BW) increased TNF-α concentration (Wang et al., 2011).
Plasma cortisol, insulin-like growth factor 1, and growth hormone concentrations
Administration of LPS increased the activity of the hypothalamic-pituitary-adrenal axis, as evidenced by increased plasma cortisol levels (Webel et al., 1997). Lipopolysaccharide challenge reduced plasma IGF-1 but no LPS challenge effect was observed for plasma GH when 200 μg/kg BW was used (Liu et al., 2003). Lipopolysaccharide challenge increased IGF-1 concentration (Wang et al., 2011).
Intestinal morphology
Jiang et al. (2009) reported that piglets injected with LPS (200 μg/kg BW) had shorter duodenum VH and decreased ileum VH:CD compared with control and which implies that LPS challenge might indirectly have affected small intestinal morphology by inducing the reduction of ADFI.
Although LPS models have been important in the elucidation of the integrated pathophysiology of infection and inflammation, this approach has limitations because it does not completely mimic the physiological changes that would occur during a bona fide infectious disease (Balaji et al., 2000). The LPS model has the following limitations: i) LPS induces a short duration response rather than a chronic immunological stress (Balaji et al., 2000) ii) pigs develop a tolerance to the multiple, subsequent LPS challenges, which was observe in the study of Kegley et al. (2001) and Liu et al. (2003), and iii) LPS and live E. coli challenge induce different immunological profiles in the weaned pigs (Zanneli et al., 2000).
Oral challenge with Salmonella enteric serotype Typhimurium
Salmonellosis is one of the most common foodborne bacteria diseases in the world (Forshell and Wierup, 2006). It is an important disease in humans and is usually associated with contaminated food, including pork products (Ojha and Kostrzynka, 2007). In addition to human health implications, Salmonella typhimurium (ST) is a pathogen of considerable importance in worldwide animal production (Forshell and Wierup, 2006). Salmonellosis in swine is one of the top 10 most common diseases in weaning and grower/finisher pigs (Haley et al., 2012).
Response criteria
Swine salmonellosis can be symptomatic or asymptomatic, and, consequently, difficult to diagnose (Rostagno and Callaway, 2012). Pigs colonized with salmonella usually exhibit varied severity and duration of fecal shedding and costly decrease in growth performance (Knetter et al., 2015). The porcine immune response to ST is also characterised largely by the local production of pro-inflammatory mediators that result in the pyrexial and neutrophil influx considered hallmarks of ST infection (Hobbie et al., 1997 as cited in Knetter et al., 2015). The response criteria that are commonly measured are discussed in detail below.
Growth performance
Balaji et al. (2000) observed that feed intake was reduced maximally in salmonella challenged pigs at 48 h and remained reduced through 120 h after challenge with 3×109 cfu/mL of S. typhimurium. Daily BW gain also was reduced during the 2 wk following infection. Turner et al. (2002) observed a reduction in ADG, ADFI, and G:F when weaned pigs were exposed to an oral challenge with 3.3×109 cfu/mL ST during wk 3 of the study. It must be noted that Turner et al. (2002) used an experimental design that did not provide an optimal test for the effects of enteric disease challenge. That is, disease-challenged pigs and uninfected pigs were housed in separate rooms which made the effects of enteric disease challenge to be confounded with room.
Rectal temperature and Salmonella typhimurium shedding
Turner et al. (2002) reported that RT of control pigs remained constant during 7 d after challenge, whereas ST challenge resulted in a distinct and expected febrile response. Rectal temperatures of ST challenged pigs were higher on d 1 to 4 after challenge but returned to levels similar to controls by d 5 after challenge (Turner et al., 2002). Rectal temperatures were elevated in ST challenged pigs relative to control pigs by 12 h, peaked at 42 h, and remained elevated throughout the remainder of the study (Balaji et al., 2000). Rectal temperature was increased in pigs challenged with 1×109 cfu/mL ST (Gebru et al., 2010). Bacteria shedding scores were higher in ST challenged pigs on d 7 after challenge and declined on d 14 post-challenge (Gebru et al., 2010). Knetter et al. (2015) reported that pigs (7 to 8 wk of age) challenged with 1×109 cfu of S. enterica serovar Typhimurium were quantitatively positive for salmonella shedding in fecal swabs while control pigs remained negative.
Plasma and serum insulin-like growth factor 1 concentration
Balaji et al. (2000) reported a marked suppression of plasma IGF-1 occurred in ST challenged IGF-1 concentrations declined from pre-infection concentrations on d 2, increased on d 4, and declined again until d 13 (Gebru et al., 2010). Circulating IGF-1 is sensitive to acute changes in feed intake and can, therefore, provide useful ancillary data as an indicator of the severity of the disease process (Burkey et al., 2004). Consistent reduction in circulating IGF-1 after ST challenge were reported in enteric disease challenged pigs (White et al., 1991). Serum IGF-1 concentration is affected in part by decreased feed intake. Individual animal differences in other factors such as GH, insulin, and thyroid hormones (Morovat and Dauncey, 1998) might contribute to changes in IGF-1 concentrations.
Acute phase proteins concentration
Salmonella typhimurium challenge produced a rise in serum haptoglobin and α1-acid glycoprotein (AGP) on d 7 after challenge but levels were comparable to controls by d 14 after challenge (Turner et al., 2002). The fact that AGP and haptoglobin levels in ST challenged pigs were higher on d 7 suggests that these acute phase proteins are appropriate indicators of the acute enteric disease experienced by infected pigs during the 7 d following ST challenge. Serum haptoglobin increased on d 6 and declined to pre-challenge concentrations on d 13 (Gebru et al., 2010).
SANITATION CHALLENGE MODEL
Le Floc’h et al. (2006) suggested that modifications of the environment and the sanitary status could be a way of stimulating an immune response of piglets reared in conventional facilities because it had been observed that unclean housing conditions expose pigs to different kinds of stressors that can lead to stimulation of the immune system, thus affecting animal performance. A number of researchers (Lee et al., 2005; Le Floc’h et al., 2006; 2009; Kahindi, 2014) have used the sanitation challenge model in studies with pigs. Lee et al. (2005) created a dirty environment by not cleaning the piglets’ shed prior to stocking and throughout the experimental period and by recycling effluent continuously beneath the floor slats in an effort to maintain increased noxious gas levels. On the other hand, the clean shed was disinfected thoroughly prior to stocking and maintained in a clean state by daily hosing of pens and aisles, flushing effluent channels with clean water and twice daily fogging the air space with a virucidal agent. Le Floc’h et al. (2006, 2009) achieved a low sanitary status by using a non-sanitized room which was neither disinfected nor cleaned after previous occupation by pigs and without antibiotic supplementation in diets. High sanitary status was achieved by using a cleaned and disinfected room with antibiotic supplementation in the diets. Kahindi (2014) achieved unclean sanitary conditions by using a room previously occupied by pigs without the room being cleaned and by adding manure slurry from a sow herd (5 kg/pen) to the expanded metal floor of 1.2×1.8 m before piglets were introduced into the room to further enhance unclean conditions. The spread of manure was repeated one week after the introduction of the piglets and the room remained uncleaned throughout the experimental period. On the other hand, clean sanitary conditions were achieved by cleaning and disinfecting the room prior to the start of the experiment and by cleaning the room once in a week throughout the experimental period.
The challenge with the use of this model is the difficult in standardizing unsanitary conditions and determine with certainty the causative agent for any immunological response observed. This makes it difficult to reproduce the model over time and among laboratories.
Response criteria
Rearing pigs under unclean sanitary conditions has been used as a model to provoke a low-grade inflammatory response. Pigs subjected to these conditions had reduced growth performance and an activated immune system that would interfere with growth because of competition between protein deposition in structural tissues and immune function (Jayaraman et al., 2015). The response criteria commonly measured in this model are discussed in detail below.
Growth performance
Lee et al. (2005) reported that pigs reared in a clean environment consumed more feed and grew faster than those housed in the dirty environment. There was no significant difference in feed conversion efficiency between the two treatments. Le Floc’h et al. (2006) reported that after 45 d of experimentation, pigs kept in bad sanitary conditions displayed depressed growth rate and feed conversion ratio compared to pigs reared in good sanitary conditions. Average final BW of pigs reared in bad sanitary conditions was reduced by 1.3 kg. ADFI was also reduced by poor sanitary conditions. In a 21 d experiment, Kahindi (2014) reported that the ADG and G:F were lower for pigs raised under unclean conditions compared with their clean counterparts during d 0 to 14 but the effect on ADG disappeared during d 15 to 21 which suggested that the ability of piglets to deal with an immunological stress improved with age and that they were able to adapt to their environment with time. However, the overall ADG was still lower for pigs raised under unclean conditions compared with those raised under clean conditions indicating that piglets were unable to fully overcome the effects of being subjected to unclean housing conditions (Kahindi, 2014; Kahindi et al., 2014). In a meta-analysis by Pastorelli et al. (2012), it was concluded that sanitary challenges negatively affect feed intake and growth, leading to a negative impact on animal well-being and economic losses. It has been suggested that the growth reduction after a sanitary challenge is not only due to the reduction in ADFI but also due to an increase in requirements related to digestive and metabolic processes (Sandberg et al., 2007).
Plasma parameters
Le Floc’h et al. (2006) reported that Plasma haptoglobin concentrations were numerically higher in high sanitary status than in low sanitary status piglets but the difference was significant only 13 days after the beginning of the experiment. Pig kept in poor sanitary conditions had greater concentrations of haptoglobin than pigs housed in good sanitary conditions (Le Floc’h et al., 2009). Plasma cortisol concentrations were significantly higher in pigs housed in dirty environment than in pigs housed in clean environment (Lee et al., 2005). Plasma IGF-1 concentrations were lower in pigs housed in dirty environment compared to those housed in clean environment (Lee et al., 2005).
OTHER CHALLENGE MODELS
Other disease challenge models that have been used in swine nutrition includes: Lawsonia intracellularis challenge (Whitney et al., 2006) and porcine reproductive and respiratory syndrome virus challenge (Boddicker et al., 2012). In cattle, disease challenge models such as bovine rhinotracheitis virus challenge (Schutz et al., 2012) and bovine viral diarrhea virus and Mannhimia haemolytica (Rose-Dye et al., 2010) have been used. Lawsonia intracellularis is the causative agent of ileitis, or porcine proliferative enteropathy (PPE) which is an enteric disease in swine. The intracellular bacteria infect immature epithelia cells located in the crypts of the villi of the intestine. Cellular proliferation and thickening of the infected intestine occur and may result in necrosis, ulceration, or hemorrhaging of the epithelial surface, or all of these (Whitney et al., 2006). Lawsonia intracellularis is present in approximately 75% of all US swine herds (Bronsvoort et al., 2001). Whitney et al. (2006) challenged pigs orally with 1.5×109 cfu/mL after a 4-wk pre-challenge feeding period. The researchers discovered that compared with unchallenged pigs, challenging pigs with L. intracellularis reduced growth rate, feed intake, and efficiency of gain and increased gauntness and diarrhea.
CRITICAL ANALYSIS OF THE EFFECTIVENESS OF FEED ADDITIVES IN PIG NUTRITION
A major challenge facing the swine industry is to identify means for controlling diarrhea in young pigs that are not only cost effective, but also suitable for sustainable pork production (Owusu-Asiedu et al., 2003). Feed additives are primarily alternative substances to the use of antibacterial agents and chemotherapeutics during weaning on the rearing farm, as they can adequately stimulate the local defensive responses, and favourably influence resident gastrointestinal microflora, but also able to improve nutrient digestion and absorption (Domeneghini et al., 2006). The feed additives to be considered in this review are zinc salts, copper salts, egg yolk antibodies, spray dried porcine plasma and mannan-oligosaccharides (MOS).
Zinc oxide
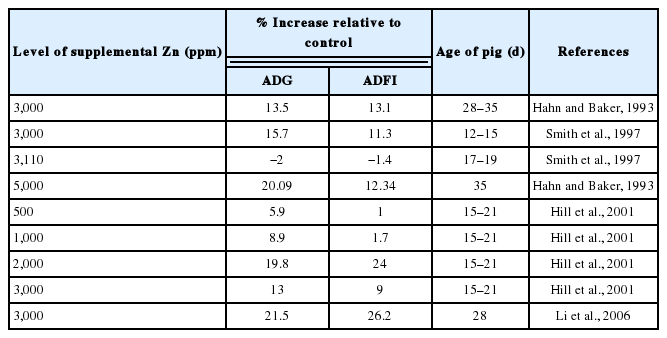
In the late 1980’s, reports began filtering into the United States from Europe that the use of pharmacological levels of zinc (Zn) as zinc oxide (ZnO), was an effective means of treating diarrhea in young pigs (Holm, 1988; Poulsen, 1989) (Table 2). A few experiments were subsequently conducted in the United States to test this theory. One of the series of studies involving high Zn diets was conducted by Hahn and Baker (1993) at the University of Illinois. Like most planned experiments, the researchers were unable to test whether ZnO was effective in preventing diarrhea scours because they did not encounter any diarrhea in any of their pigs, including the controls. However, they did find that in each of the 3 experiments, ZnO stimulated growth rate of the pigs. Averaged across the 3 trials, ZnO supplementation at 3,000 and 5,000 ppm increased daily weight gains by 17% and 20%, respectively and daily feed intakes by 14% and 12%, respectively (Hahn and Baker, 1993). Also, ZnSO4 supplementation at 3,000 and 5,000 ppm increased daily weight gains by 16.6% and 1.5%, respectively, and daily feed intakes by 9.7% and 5.42%, respectively. At concentrations greater than 1,000 ppm, plasma Zn increased linearly for all 3 sources of Zn (ZnO, ZnSO4, Zn-Lys complex). Plasma Zn of pigs fed ZnO was only 55% of the value obtained from pigs fed ZnSO4 whereas plasma Zn of pigs fed Zn-Met was 120% of the plasma Zn value obtained from pigs fed ZnSO4 (Hahn and Baker, 1993). This observation shows that pigs utilize Zn from ZnO far less efficiently than Zn from ZnSO4.
Evidence from Wedekind and Lewis (1993) also demonstrated that bone Zn accumulation in pigs fed ZnO is only 67% to 70% as great as that in pigs fed ZnSO4. Thus, plasma Zn concentration in pigs fed high levels of added Zn (between 1,000 and 5,000 mg of Zn/kg) may represent a useful and non-invasive means of assessing Zn bioavailability of various inorganic Zn sources (Hahn and Baker, 1993).
Smith et al. (1997) reported increase in ADG and ADFI when pigs were fed 3,000 ppm Zn from ZnO. Specifically, compared to the basal diet, supplementing pigs’ diet with 3,000 ppm of Zn from ZnO led to 27% increase in ADG and 17.7% increase in ADFI (Smith et al., 1997). Both traditionally weaned and early weaned pigs fed 3,000 ppm of Zn had higher ADG and plasma and liver Zn concentrations for the entire 28-d period compared with pigs fed adequate Zn (Carlson et al., 1999).
Other researchers (Carlson et al., 1995; Poulsen, 1995; Smith et al., 1997) have reported a range of 10% to 26% increase in growth performance when nursery pigs are fed pharmacological (2,500 to 4,000 ppm) concentrations of Zn. Poulsen (1995) reported that a supplement of 2,500 ppm Zn as ZnO fed to weanling pigs for 2 weeks reduced the incidence and severity of non-specific PWD by up to 50%. Researchers from 10 Universities in the United States cooperated in a study to investigate the impact of pharmacological dietary Zn on performance of weanling pigs with diverse health status and housing conditions. Improved daily growth (15%) and feed intake (11%) were found with 3,000 ppm Zn in diet (Hill et al., 1996).
Another cooperative study involving 9 University research stations in the United States evaluated the effects of different dietary ZnO concentrations (0, 500, 1,000, 1,500, 2,000, and 3,000 mg Zn/kg) on the performance of post-weaning pigs and reported that weight gains, feed intakes, and gain:feed ratios increased by approximately 10%, 5%, and 5%, respectively, when pigs were fed 2,000 mg Zn/kg diet compared with those that were fed basal diets (Hill et al., 2001). The results clearly demonstrated a growth response to added ZnO that reached a plateau at dietary concentrations of 1,500 to 2,000 mg Zn/kg.
Also, Li et al. (2006) reported that adding 3,000 mg Zn/kg in the form of ZnO to the diet of weanling pigs increased daily BW gain by 21.5% and daily feed intake by 26.2%. A new and important finding from the study of Li et al. (2006) was that dietary Zn supplementation increased VH of the piglet by 13.5%. This provides a useful model with which to test the hypothesis that dietary supplementation with high levels of Zn improves intestinal structure and function by increasing intestinal expression of the IGF-1 and IGF-1R genes (Li et al., 2006).
There is much speculation about the mechanism responsible for the effect of high levels of ZnO in promoting piglet performance. The Zn ion is considered to be a major causative factor on the basis of the observation that a higher bioavailability of Zn was associated with a better growth-promoting effect (Hahn and Baker, 1993). However, ZnO, whose bioavailability is much lower than that of ZnSO4 or zinc-methionine complex (Zn-Met) (Wedekind et al., 1994) had a stronger growth-promoting effect than ZnSO4 or Zn-Met (Hahn and Baker, 1993; Wedekind et al., 1994).
Furthermore, Mavromichalis et al. (2000) found that both high (93%) and low (39%) bioavailability of ZnO had the same performance-enhancing effect in piglets. These findings indicate that the bioavailability of a high level of Zn in the diet is not a major factor affecting growth performance in piglets. It was noted in recent times that the use of high doses of inorganic Zn has raised concern due to elevated Zn in feces when fed for more than 10 d (Rincker et al., 2005). Therefore, Castillo et al. (2008) evaluated the efficacy of a source of organic Zn (Bioplex Zn) to enhance growth performance, gastrointestinal health, and immune response in weaned pigs. The trial of Castillo et al. (2008) was based on the hypothesis that if the mode of action of Zn is based on a systematic effect, an organic form of Zn with greater bioavailability would allow reduced concentration in feed and subsequent release in the environment while maintaining benefits to the animals. It was found that the use of Bioplex Zn improved G:F in the starter period, improved fecal scores for the first 21 d, decreased enterobacteria counts in the jejunum, but had no effect on the serum concentration of the immunoglobulins and ileal IgA in the jejunum (Castillo et al., 2008). Supplementing ZnO increased serum Zn, which reduced serum P and Ca, indicating Ca-Zn-P precipitation. In addition, phytase increased serum Ca, but only in the absence of Zn, further indicating a complex interaction between Zn, Ca, and P in the blood (Walk et al., 2012).
Copper sulphate
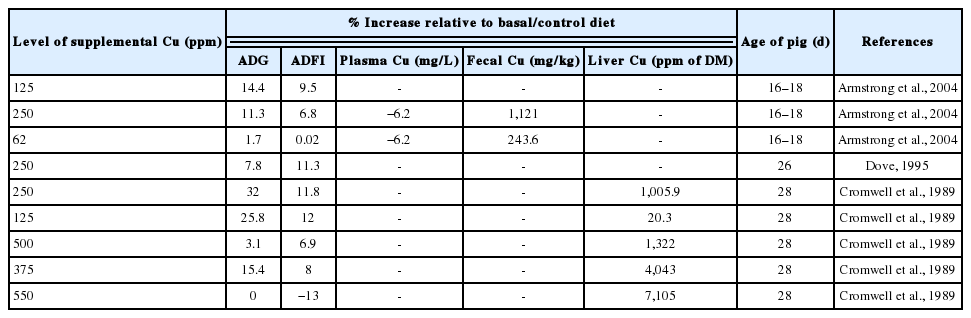
Copper is a unique mineral element in that it acts as a growth stimulant in swine when fed at high dietary levels (Cromwell et al., 1989). The sulphate salt of Cu is the most common source used in feeds for growth promotion (Table 3). It is routinely supplemented to weanling pig diets at concentrations above the nutritional requirement of the animal. Cromwell et al. (1989) evaluated the efficacy of various levels (0, 30, 125, 250, 375, and 500 ppm) of dietary Cu as a growth promoter for weanling pigs. Dietary inclusion of 250 ppm level of CuSO4 improved growth rate by 24% to 39% and improved efficiency of feed utilization by 11% to 21%. Feed intake also was increased by 8% to 17% in pigs fed this dietary level. The magnitude of the improvement in growth performance was diminished when CuSO4 was increased to 375 ppm and was completely lost when diet was increased to 500 ppm Cu.
Dove (1995) in a nutrient balance study with weanling pigs, evaluated the effect of Cu supplementation on the ability of nursery pigs utilize nutrients from diets containing 0 or 5% supplemental animal fat. He found that pigs fed diets with 5% added fat and 15 ppm Cu had increased growth rate and feed efficiency compared with pigs fed 0% fat and 15 ppm of Cu diet or pigs fed the 0% fat and 250 ppm of Cu diet. ADFI was increased by the addition of 250 ppm of Cu to the diet. Pigs fed 250 ppm of Cu consumed 15.7% more feed than did pigs fed the 15 ppm of Cu diet. This is a direct reflection of the increased gain and feed efficiency observed in these pigs because pigs were fed based on BW. The addition of Cu increased the apparent digestibility of fat at both levels of fat indicating that Cu played a role in the digestion of animal fat.
Studies with finishing pigs indicate that Cu increases the level of unsaturated fatty acids in backfat (Amer and Elliot, 1973). Ho and Elliot (1974) suggested that Cu enhanced the specific activities of the fatty acyl desaturase systems, thus altering the composition of the depot fat. Armstrong et al. (2004) determined the efficacy of copper source (copper citrate and CuSO4) and dietary concentration on growth performance and fecal Cu excretion in weanling pigs, as well as the effect on odor characteristics of swine waste. They observed that feeding 250 ppm Cu as CuSO4 improved growth rate and feed efficiency over the 45 d nursery period.
Of further interest in the study of Armstrong et al. (2004) was that lower dietary Cu concentrations (125 ppm), regardless of source, were as effective at stimulating gain as 250 ppm Cu from CuSO4. In agreement with this data, 100 to 125 ppm Cu has been shown to improve growth performance at rates similar to 250 ppm Cu (Roof and Mahan, 1982; Coffey et al., 1994). Previous research has indicated that pigs normally excrete 70% to 95% of the Cu consumed in diets (Kornegay and Harper, 1997). This poor retention of Cu by the pig when pharmacological concentrations of Cu are fed presents an environmental concern, because excess Cu in swine feces results in Cu accumulation in the soil (Armstrong et al., 2004). There was no effect of dietary Cu, regardless of Cu source on the odor characteristics of swine waste even though previous research has demonstrated that Cu supplementation to nursery diets improved the odor characteristics of swine waste (Armstrong et al., 2000).
Egg yolk antibodies
Oral administration of antibodies derived from serum and colostrum and even with monoclonal antibodies has been very successful. Of particular veterinary interest is the use of chicken egg yolk antibodies for the treatment of porcine colibacillosis (Yokoyama et al., 1992) (Table 4). Vaccination of laying hens provides a cheaper and good alternative antibody source; the eggs are collected after a high level of antibodies is reached in the egg yolk (Yokoyama et al., 1992). Yolken et al. (1988) suggested that egg preparations might serve as source of antiviral antibodies for humans. The first clinical use of spray dried chicken egg yolk antibodies against ETEC infection in piglets was first described by Yokoyama et al. (1992). The different therapeutic regimens with various titers (0, 156, 625, and 2,500) of anti-K88, -K99, and -987P antibodies reduced considerably the severity of diarrhea in piglets receiving high-titer antibodies (Yokoyama et al., 1992). On the basis of their findings, the antibody titer of 2,500 was considered the most efficacious in protecting piglets from the proliferation of ETEC strains in the intestinal tract and its harmful consequences.
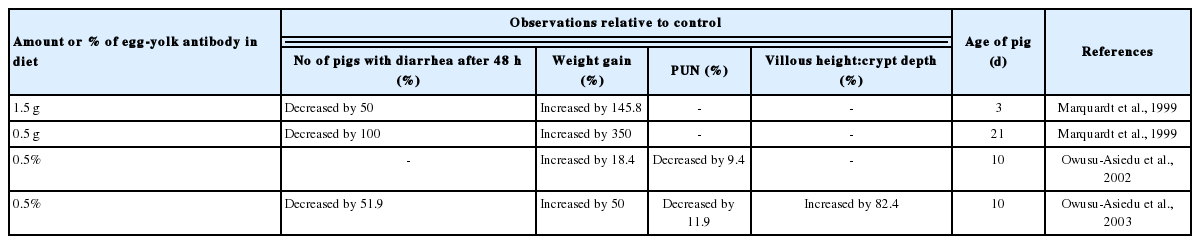
Effect of feeding egg-yolk antibody on pigs’ growth performance, plasma urea nitrogen, intestinal morphology and protection against diarrhea
Jin et al. (1998) demonstrated in an in vitro study that purified antibody from the yolk of chicken immunized against E. coli (K88) was able to block the binding of ETEC K88 to the mucosal receptor and prolonged incubation of egg-yolk antibodies and that ETEC did not further reduce the degree of adhesion of ETEC to mucus and that egg-yolk antibodies were unable to displace ETEC once the bacteria were attached to the mucus of the small intestine.
Marquardt et al. (1999) demonstrated that egg-yolk antibodies protected both neonatal and 21-d-old weaned piglets from diarrhea induced by a challenge with E. coli K88+ MB. Owusu-Asiedu et al. (2003) demonstrated that egg-yolk antibody has important therapeutic and prophylactic value in the diets of early-weaned pigs by controlling and preventing enterotoxigenic E. coli infection, thereby improving pig performance.
Also, Owusu-Asiedu et al. (2003) assessed the effectiveness of egg yolk antibodies (0.3% and 0.2% egg yolk powder containing specific anti-K88 and anti-F18 antibodies, respectively) in controlling ETEC relative to conventional additives in early weaned pigs fed pea protein isolate based diets. Piglets fed egg yolk antibodies recovered 3 d after ETEC challenge indicating that the presence of the specific anti-ETEC antibody binds the E. coli, thereby preventing colonization and proliferation, resulting in subsequent removal of the E. coli.
Spray-dried porcine plasma
Spray-dried porcine plasma (SDPP) has been used widely in the diet of early weaned pigs since it has been shown to greatly improve weight gains (10% to 50%) as a result of increased feed intake (Coffey and Cromwell 1995; Jiang et al., 2000). It has been shown that the feeding of SDPP reduces the incidence and severity of diarrhea in young pigs (Van der Peet-Schwering and Binnendijk, 1995) (Table 5). It was reported by Frank et al. (2003) that pigs fed diet containing 7% spray dried plasma had a 52 g/kg reduction in G:F compared to the pigs fed diet with no spray dried plasma.
Bosi et al. (2004a) demonstrated that feeding piglets a diet containing spray-dried plasma improves growth and protects against ETEC K88 infection by maintaining mucosal integrity, enhancing specific antibody defense, and decreasing inflammatory cytokine expression in intestine. The data of Bosi et al. (2004b) also provided support that SDPP is a better source of protein than fish for weanling pigs. Results from Gao et al. (2011) demonstrated that spray dried animal plasma (SDAP) addition increased ADG and ADFI by 24.3% and 21%, respectively, whereas G:F was not affected. These findings are consistent with the results which reported that mean SDAP-induced changes in ADG, ADFI, and G:F in the first 2 wk for pigs weaned at a postnatal age greater than 4 d were 26.8, 24.5, and −3.2, respectively (van Dijk et al., 2001).
Autoclaved SDAP was reported to improve ADG and ADFI by 15% and 18%, respectively (Gao et al., 2011). The improvements of ADG and ADFI by autoclaved SDAP which were less than those by SDAP indicated that the heat-sensitive immunoglobins only partially contributed to the benefits of SDAP. Owusu-Asiedu et al. (2002) reported that autoclaved SDAP decreased feed intake and ADG, which was inconsistent with the results of Gao et al. (2011). However, the results of Owusu-Asiedu et al. (2002) plus those of others and results from Gao et al. (2011) indicate that factors other than immunoglobins, including growth factors, cytokines, and other unknown compounds, in SDAP could have physiological impacts (Moreto and Perez-Bosque, 2009).
Peace et al. (2011) evaluated the effects of 2.5% and 5% dietary inclusion levels of SDPP on post-weaning intestinal barrier function, mucosal inflammation, and clinical indices of gut health in pigs. The researchers demonstrated that dietary inclusion of 2.5% and 5% SDPP reduced ileal and colonic paracellular permeability of 14C-inulin and reduced ileal 3H-mannitol compared with controls. The 5% SDPP diet reduced colonic short-circuit current, an index of net electrogenic ion transport, and fecal scores. This demonstrated a beneficial role of SDPP inclusion in ameliorating intestinal barrier dysfunction, mucosal inflammation, and diarrhea observed during the post-weaning period in pigs (Peace et al., 2011).
Mannan-oligosaccharides
Prebiotics which were first identified and named by Gibson and Roberfroid (1995) are non-digestible food ingredients, which stimulate the growth or activity or both of bacteria in the digestive system. In swine nutrition, prebiotics, probiotics, and sybiotics seem to be functional components with beneficial effects on growth performance, gastrointestinal function and health. LeMieux et al. (2003) reported that MOS has been used as prebiotics in livestock feeds. Mannan-oligosaccharides consist of a mannan and a glucan component (Table 6). The structure of the mannan component resembles that of the carbohydrates on the gut wall. Mannan-oligosaccharides derived from yeast cell wall may offer an alternative to traditional ingredients (such as antibiotics, Cu, and excess Zn) resulting in equal or better growth performance of nursery pigs (Davis et al., 2002). Fructan supplementation improved growth performance and apparent total tract digestibility (ATTD) of dry matter (DM) and gross energy (GE), improved the fecal microbial balance, and inhibited the fecal E. coli. Furthermore, fructan may decrease fecal noxious gas emissions by finishing pigs (Zhao et al., 2103).

Effects of mannan oligosaccharides (MOS) on growth performance, diarrhea score and serum IgG of weanling pigs
The MOS has the ability to influence the microbial population in the intestinal tracts (Davis et al., 2002). This modification is accomplished by the ability of MOS to attach to mannose binding proteins on the cell surface of some strains of bacteria, thereby preventing these bacteria from colonizing the intestinal tract by interfering with the binding of carbohydrate residues on epithelia cell surfaces (Spring et al., 2000). Dietary supplementation with 0.10% levan-type fructan can improve growth performance, digestibility, and fecal Lactobacillus counts, and has a beneficial effect on the immune response during an inflammatory challenge in growing pigs (Li and Kim, 2013).
The results of Davis et al. (2002) indicated that the addition of MOS to swine diets moderately improved weight gain and feed efficiency. However, the magnitude of response was not as great as that observed with pharmacological additions of Cu. White et al. (2002) also reported that yeast (containing 5.2% MOS) reduced colonization of total coliforms in the duodenum, jejunum, cecum, and colon but did not have a consistent effect on colonization of E. coli K88. Pigs fed yeast tended to have higher serum IgG levels than controls (White et al., 2002). LeMieux et al. (2003) reported that addition of MOS to weanling pig diets increased growth performance during phase 2 of the nursery period but only when an antibiotic was included in the diet and when excess Zn was not included. Thus MOS may have the potential to replace excess Zn that is commonly added to nursery pig diets (LeMieux et al., 2003). Dietary supplementation of chito-oligosccharide (COS) appeared to increase egg production and quality by increasing nutrient digestibility. Additionally, COS improved WBC and total protein concentration in laying hens (Meng et al., 2010).
Zhao et al. (2011) reported that pigs fed MOS diet for 28 days had greater ADG than pigs fed the negative control (basal without antibiotic). Pigs fed the positive control (basal+antibiotic) and MOS diets had increased ADFI compared with pigs fed the negative control diet. On d 14 of the experiment, the ATTD of DM and n in pigs fed the positive control and MOS diets were greater that the pigs fed the negative control diet (Zhao et al., 2011). Diarrhea score in pigs fed the MOS diet was reduced compared with pigs fed the negative control diet (Zhao et al., 2011). Addition of a dietary carbohydrate mixture (α-galactosidase and β-mannanase 0.05%) could improve growth performance and nutrient digestibility of finishing pigs (Kim et al., 2013).
CONCLUSION
Various disease challenge models that have been used in animal nutrition include PWD using specific pathogenic strains of ETEC, bacteria LPS challenge, oral challenge with Salmonella enteric serotype Typhimurium, sanitation challenge and other challenge models such as Lawsonia intracellularis challenge. For oral ETEC challenge, varying volume (1.5 to 6 mL) and varying concentrations (109, 6.3×109, 1010 and 1012 cfu/mL) of ETEC reduced growth performance and caused mild to severe diarrhea. For LPS challenge, a concentration of 5 to 200 μ/kg BW reduced growth performance, elevated PUN, altered intestinal morphology and increased plasma IGF-1 and TNF-α concentrations. For salmonella challenge, varying concentrations (109, 3×109, and 3×109 cfu/mL) of ST caused reduction in growth performance and elevated RT. The sanitation model of immune challenge has proven to be effective when growth performance and PUN were used as response criteria, although this model does present significant challenges in terms of repeatability.
Feed additives such as ZnO, CuSO4, spray-dried porcine plasma, egg-yolk antibodies, and MOS have been used by various researchers to improve growth and also to prevent diseases. The effectiveness of these additives depends majorly on the amount added to the diet. Above 1,000 ppm of ZnO and 100 ppm of CuSO4, growth performance is enhanced while improvement in growth performance is lost above 3,000 ppm of ZnO and 250 ppm of CuSO4. Addition of 0.1% to1.5%, 10%, and 0.1% to 0.2% of egg-yolk antibodies, SDPP, and MOS, respectively, were effective in enhancing growth performance and preventing diarrhea in weanling pigs.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.