INTRODUCTION
Large-scale livestock breeding has become a serious social problem with respect to the storage and treatment of livestock manure. The waste generated in the process of handling livestock manure contains a large amount of nitrogen and phosphorus, which are the main agents causing eutrophication. If the waste is discharged or an excessive amount is used as a fertilizer on soil without proper treatment, environmental problems can occur. Solid and liquid composting are widely used as resource recovery methods for the disposal of domestic livestock manure, and biological treatment is predominantly used for the purification of urine and wastewater. However, because the soil is not sufficiently secured for distributing fertilizers, it is desirable to produce safe and high quality fertilizers by decomposing the manure and storing or transporting it for future use. Treatment costs of livestock manure with water contents above 97% are high because it is necessary to mix a large amount of sawdust or husk. Moreover, this manure is highly likely to become sawdust compost, in which the content of the fertilizer active ingredient is low. In addition, during the manufacturing of liquid fertilizer, highly concentrated ammonium is present, and therefore, the fertilizer is strongly aerated to remove the bad odor. Accordingly, most of the ingredients undergo nitrification to produce the content of the fertilizer active ingredient low. Further, it is difficult to handle and transport the fertilizer because it is liquid. The fertilization period is fixed according to the state of the domestic farming industry, and thus, it is difficult to spray fertilizers in autumn and winter. Thus, tremendous amounts of liquid fertilizer are stored. Although there is a necessity for the recovery of livestock resources, civil complaints have been filed due to the odor emanating from the treatment process; the product is also a low quality fertilizer, with low added value, and thus becomes a nuisance.
Unless the quality of life is improved and meat consumption is reduced, domestic livestock farming will continue to increase. Recovering the energy in manure is a good method of disposing the constantly increasing livestock manure more effectively. Currently, the method mostly used for this is the production of biogas through anaerobic digestion. However, this method creates problems related to disposing the solid and anaerobic digestion wastewater that remains after energy recovery.
Anaerobic digestion fluid contains more highly concentrated nitrogen and phosphorus than ordinary livestock wastewater, and thus, it is very difficult to meet the reinforced water quality standard for anaerobic digestion fluid. Anaerobic digestion liquid can cause bad odor and contains high-molecular substances that have chromaticity. Thus, if this liquid flows into a river or lake without treatment, the increase in chromaticity could cause a decrease in the transillumination of the aquatic ecosystem, which could lead to secondary problems including degradation of the self-purification capacity of the ecosystem and the reproduction of pathogenic bacteria. Due to such problems, most treatment facilities face difficulties in disposing of highly concentrated nitrogen and phosphorus in anaerobic digestion liquid. If the organic fertilizer ingredients, which are largely contained in the livestock wastewater or anaerobic digestion fluid, are powdered or solidified, they can be conveniently transported to other regions. It would also be possible to use them all-year round like chemical fertilizers and this would reduce the amount sprayed on farmlands; thus, it is thought that there would be an effect of reducing the number of workers. Further, when the fertilizer used in the farmland is in the form of urea, only 40% of N is used as fertilizer by plants and the rest is swept away in various forms (Liang et al., 2007); a large amount (26.5% to 29.4%) is also discharged as greenhouse gases such as N2O. Since the heat absorption rate of N2O is higher than that of CO2, N2O contributes to global warming by more than about 300 times that of CO2 (Hallett, 2002). When struvite is used as a fertilizer, the plant absorbs all the N due to struvite’s slow releasing characteristics (Lee et al., 2009) and this can also minimize N2O emissions (Chu et al., 2007).
In the Netherlands, in order to recover and recycle phosphorus from livestock manure, manure is completely incinerated and the ashes are recycled (Willem et al., 2001). Ren et al. (2014), Enhanced adsorption of phosphate by loading Nanosized Ferric Oxyhydroxide on anion resin. Currently in South Korea, the source materials needed in the phosphorus-related industry are imported from other countries. The exhaustion of phosphorus is a global concern, and, therefore, securing sources for replacing phosphorus is an urgent problem (Moriyama et al., 2001; Frank and Mark, 2009). As a practical measure to this problem, struvite crystallization, which is a chemicophysical treatment method, is mainly being studied, and the phosphorus generated and recovered through such processes is available as high-priced fertilizer in some countries including Japan (Moriyama et al., 2001; Ueno and Fujii, 2001).
Struvite, generally called guanite or magnesium ammonium phosphate (MAP), is combined with a NH4+:Mg2+:PO4− mole ratio of 1:1:1. Struvite crystallization has advantages in that it can process highly concentrated nitrogen and phosphorus at the same time, the detention period is short, there is no need for another facility, and the site area can be reduced (Jo et al., 2003; Kim et al., 2006; Weon et al., 2009). In particular, the wastewater generated after the thermophilic digestion contains highly concentrated phosphorus and thus can reduce the injection volume of outer phosphorus (PO43−) that is required in the crystallization. The subjects of struvite studies until now have included livestock wastewater, sewage water, and anaerobic digestion fluid. The concentration of early total chemical oxygen demand (TCOD) values and NH4+-N of the studied wastewater was about 2,700 mg/L and 1,700 to 2,000 mg/L on average. However, the treatment of TCOD and NH4+-N in anaerobic digestive fluid of swine wastewater containing highly concentrated nitrogen is a significant problem with respect to factors such as recovery rate.
Thus, in this study, struvite crystallization was conducted using thermophilic aerobic digestion sludge fluid of swine manure containing 17,125 mg/L of TCOD, 3,534 mg/L of total Kjeldahl nitrogen. During the crystallization reaction, the influence of factors affecting the treatment of highly concentrated ammonia nitrogen and phosphorus such as pH, mixing time (td) and mixing intensity (G), injection volume of magnesium and phosphorus, and temperature were analyzed.
MATERIALS AND METHODS
Wastewater characteristics
The wastewater used in this study comprised the supernatant of anaerobic digestive fluid of swine manure containing highly concentrated nitrogen and phosphorus. The characteristics of the wastewater are shown in Table 1; the average concentration of ammonia nitrogen and phosphate phosphorus was 3,400 mg/L and 1,342 mg/L, respectively. The ratio of the ammonia nitrogen to the total nitrogen of the subject wastewater was over 96%, and the ratio of the phosphate phosphorus to the total phosphate was over 77%, confirming that the wastewater was appropriate for struvite crystallization. T-COD of this anaerobic digestive fluid was 17,125 mg/L, which was very highly concentrated nitrogen containing wastewater.
Experimental materials
A 1,000 mL beaker was used in the experiment and the amount of wastewater was set to 900 mL. The reagent used in the struvite crystallization was MgCl2·6H2O. Every experiment for the struvite crystallization reaction of anaerobic digestive fluid of swine manure was conducted by placing 1,000 mL of digestion fluid in a 2,000 mL Erlenmeyer flask at various temperatures, pH, and mixing speed. Except for special circumstances, the digestion fluid was centrifuged (10,000 rpm, 10 min) and then the supernatant was used for the experiment at room temperature and 100 rpm. The pH of the digestion fluid was controlled by adding 5 N NaOH before the addition of MgCl2·6H2O, and by mixing the fluid through the impeller. Experimental conditions were shown in Table 2.
The influence of initial pH during struvite crystallization
To observe the influence of pH during the struvite crystallization reaction, 1,000 mL of anaerobic digestion supernatant was placed in the prepared beaker and evenly mixed at 100 rpm. After measuring the initial pH and temperature, magnesium and phosphate phosphorus were injected to obtain a Mg2+:PO43− mole ratio of 1:1. The initial pH of the wastewater was 8.3, and thus the pH was set to 9, 10, 11, 12, and 13 by adding 5 N NaOH after injecting magnesium. After the struvite crystallization reaction, the volume of the precipitates was measured 10 minutes after precipitation to check the treatment efficiency of ammonia nitrogen and phosphorus after the crystallization. Then, the obtained supernatant was filtered with 0.45 μm syringe filters.
Influence of mixing time and intensity during struvite crystallization
During the struvite crystallization reaction, the mixing time was set to 10 minutes, 60 minutes, and 24 hours, and the intensity was variously set to 100 rpm and 200 rpm. Here, the reaction pH was not adjusted, as the phosphate removal efficiency in a previous experimental result was similar in the range from 9 to 13. After the struvite crystallization reaction, the volume of the precipitates was measured after 10 minutes, 1 hour, and 24 hours of precipitation to check the treatment efficiency of ammonia nitrogen and phosphorus after the crystallization. After that, the mixing intensity increased to 200 rpm for 24 hour reaction. In this reaction, magnesium and phosphate phosphorus mole ratio (Mg2+:PO43−) was 1:1.5, which has been determined to be the optimal mole condition as it resulted in the maximum phosphate removal efficiency in a previous experimental result. Then, the obtained supernatant was filtered and analyzed with 0.45 μm syringe filters.
Optimal mole ratio of Mg2+ and PO43-P
In this study, a large amount of NH4+-N was present in the wastewater making it impossible to obtain the ratio of 1:1:1 that is generally used in the process of crystallizing struvite. Thus, with a focus on the recovery of phosphorus, the optimal injection amount of Mg2+ and PO43-P was determined. After preparing 1,000 mL of the subject wastewater, the injected mole ratio was altered by setting 1 as the reference point in order to derive the optimal injection amount. Here, the pH not adjusted before injecting Mg and the reaction was conducted at room temperature at 100 rpm for 1 hour.
Analysis method
Analysis of every sample used in this study was conducted after filtering the samples with 0.45 μm syringe filters in order to remove the floating materials in the samples. The ammonia nitrogen was measured using a HACH DR-5000 and analyzed using the Salicylate method in the HACH DR-4000 manual (DR5000, HACH Inc., Loveland, CO, USA). Phosphorus was analyzed by using the Molybdovanadate method.
The pH meter (Orionstar, ThermoScientific, Walthem, MA, USA) was calibrated after each experiment. After struvite crystallization, the precipitates were dried at room temperature, and the type and shape of the crystals were identified through X-ray diffraction (XRD) analysis (using the X-ray diffractometer of Advance D8, Bruker Co., Billerica, MA, USA) and standard error of the mean (scanning electron microscopy) measurements (using JSM-5600, JEOL Co., Tokyo, Japan).
RESULTS AND DISCUSSION
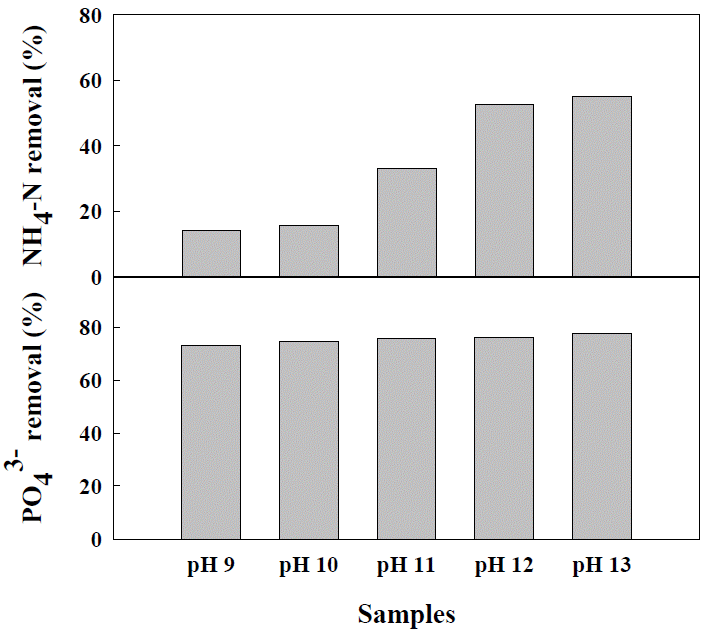
Influence of early pH on struvite crystallization
Factors such as the concentration of Mg2+, ratio of Mg2+, NH4+, and PO43−, pH, temperature, aeration rate, and presence of Ca2+ significantly affect struvite crystallization (Yetilmezsoy and Zengin, 2009). In particular, pH is extremely important. Generally, the solubility of struvite increases according to increases in pH, and thus it is desirable to induce crystallization by maintaining a high pH in order to remove nitrogen and phosphorus. The influence of pH change in the struvite crystallization reaction of anaerobic digestion fluid with an average concentration of ammonia nitrogen is shown in Figure 1. As shown in the figure, the removal efficiency of ammonia nitrogen has a wide range, from pH 9 to pH 13. However, ammonia nitrogen was more effectively removed in an environment with pH over 12. The removal efficiency was 33.1%, 52.6%, and 54.9% at pH 11, 12, and 13, respectively. Previous studies have reported that the most appropriate pH for struvite crystallization of swine manure and sewage water is alkaline condition such as 8 to 12 (Lee et al., 2010; Rahman et al., 2011; Ye et al., 2011; Hutnik et al., 2013).
A removal of phosphorus was observed in wide range of pH from 9 to 13 (Figure 1) in this research. The removal efficiency was 73.0%, and 77.8% at pH 9, and 13, respectively. With the increase in pH, the solubility of struvite decreased, but it has been reported that the added Mg2+ forms coagulations such as Mg(OH)2, which cause relatively low NH4+-N removal efficiency (Choo et al., 2011). The pH is an extremely significant factor in struvite crystallization, and in consideration of the ratio of the chemicals, it would be most effective to remove phosphorus and nitrogen through only a simple mixing under weak alkaline conditions.
Influence of mixing time and intensity during struvite crystallization
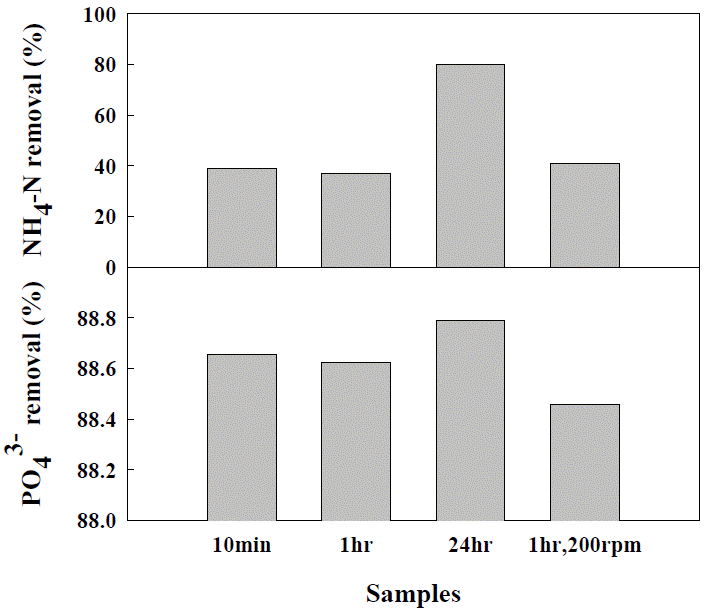
During struvite crystallization, aeration rate plays an important role in removing ammonia. Air flow volatilizes and removes dissolved ammonia through mixing in the wastewater. Moreover, air flow becomes the driving force, which dilutes the concentration of gas-phase NH4+-N and separates the dissolved NH4+-N from the gas-phase. It has been reported that sufficient aeration time can enable the effective removal of ammonia (Yetilmezsoy and Zengin, 2009). Yetilmezsoy and Zengin (2009) reported that NH4+-N in the reactor could be removed by up to 95.3% by mixing at a speed of 0.6 L·m−1 for 24 hours (Yetilmezsoy and Zengin, 2009). Liu et al. (2011b, c) stated that the formation of struvite in the reactor is proportional to the aeration rate, and that it reaches a plateau at a speed of 0.73 L·m−1. According to Suzuki et al. (2007) and Battistoni et al. (1997), the pH of wastewater increased with the aeration because of CO2 stripping (Suzuki et al., 2007). They also stated that at an aeration rate of 12 m3·h−1, the pH was within 7.5 and 8, and that at an aeration rate of 16 m3·h−1, the pH increased from 8.0 to 8.5. According to Choo et al. (2011), struvite crystallization is not largely affected when the mixing time is set between 1 min and 30 min and when the mixing intensity is set to over 85 s−1. Kim et al. (2006) stated that the NH4+-N and PO43-P removal efficiency was 75% and 89% within 5 minutes, and 82.1% and 96.8% at 10 minutes, respectively, and that the efficiency did not increase after 10 minutes (Kim et al., 2006). Although there was slight difference in the time of arriving at the maximum efficiency, they inferred that the maximum efficiency was arrived at within a short time span of between 0 min and 30 min.
In this study, NH4+-N showed 37% removal under the condition of 1 h, and then, it increased to 80% for 24 h reaction (Figure 2). It was considered because of the nitrogen (CO2) stripping effect. In the case of PO43-P, the increasing reaction time from 10 min to 24 h did not have a large influence on the crystallization (about 88% removal) and changing the mixing speed between 100 rpm and 200 rpm had the same result.
Optimal injection amount of Mg2+
It has been reported that, in general, for NH4-N, COD, and chromaticity removal, there is no major difference when using MgSO4·6H2O or MgCl2·6H2O as the samples for struvite crystallization (Yetilmezsoy and Zengin, 2009). However, it has been reported that when MgO is used, Mg(OH)2 raises the total suspended solid and, thus, reduces the NH4-’N removal efficiency. However, the Ca2+ in the wastewater reacts with PO4 generating substances such as hydroxyapatite, dicalcium phosphate, and octacalium phosphate. Thus, it has been reported that the generation of calcium phosphate inhibits struvite crystallization (Doyle and Parsons, 2002).
The ratio of PO43-P:Mg2+ that is known to be appropriate for struvite crystallization is either 1:1 or 1:1.2 (Rahman et al., 2011). According to Yetilmezsoy and Zengin (Yetilmezsoy and Zengin, 2009), the N and COD removal rate is lower when the ratio of Mg2+:NH4+:PO42− is lower (0.5:1:1, 0.8:1:1, 1:1:0.5, 1:1:0.8) than the high mole ratio (1.2:1:1, 1.5:1:1, 1:1:1.2, 1:1:1.5).
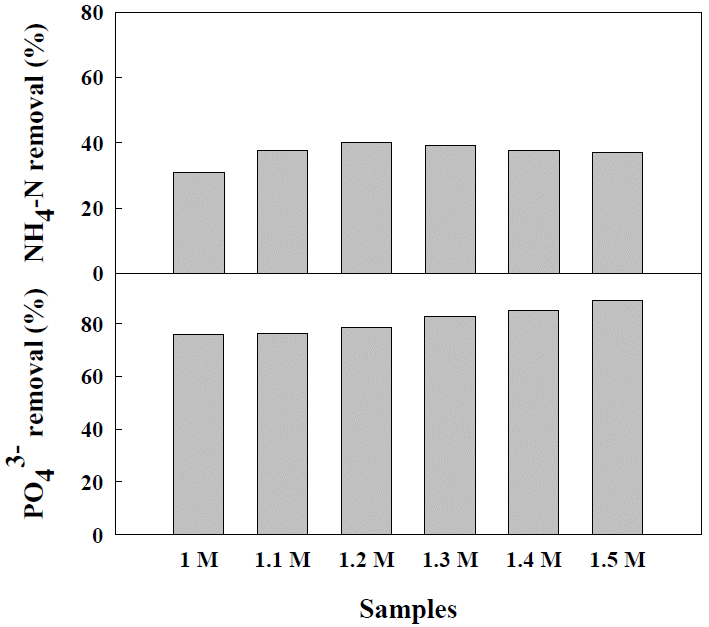
The concentration ratio of NH4+-N and PO43-P was approximately 30:1 in the livestock wastewater, and therefore, the phosphorus removal efficiency was high while the ammonia removal efficiency was low. In consideration of such characteristics of anaerobic digestive fluid of swine manure, the PO43-P dissolved in the wastewater was taken as the reference point in this study in order to determine the addition ratio. Accordingly, PO43-P: Mg2+ was altered to be 1:1, 1:1.1, 1:1.2, 1:1.3, 1:1.4, and 1:1.5, and then the removal efficiency of NH4+-N and PO43-P was measured (Figure 3). As a result, the NH4+-N removal efficiency (40%) was highest when 1.2 M of Mg2+ was added. In the subsequent concentration, the removal efficiency showed a tendency to decrease. The PO43-P removal efficiency increased from 75% to 88% as the amount of added Mg2+ increased from 1.0 M to 1.5 M, respectively.
It has been reported that although all the PO43-P participates in the crystallization of struvite at the optimal injection amount, above that level it forms substances such as hydroxyapatite by reacting with other spilt or dissolved ions (Dorozhkin, 2010; Capdevielle et al., 2013). There are several studies reporting that, generally, in the crystallization of struvite, an injection mole ratio of NH4+:Mg2+:PO43− higher than 1:1:1, which is the theoretical mole ratio, is necessary. This appears to result from the consumption of injected Mg due to the crystallization of substances such as calcium dissolved in the wastewater through reaction with substances such as PO43−-P, or the formation of amorphous precipitate or complex compounds such as Mg(OH)2 and MgHPO4·3H2O (Schulze-Rettmer, 1991).
The optimal mole ratio can differ according to the type and characteristics of the wastewater used in the struvite crystallization because of the variation of the dissolved NH4+-N and PO43-P concentrations. The theoretical optimal value of 1:1:1 cannot be achieved because the NH4+:PO43− ratio of wastewater cannot be 1:1, and, thus, when the PO43-P concentration is lower, the values of the variables in the reaction can vary. Generally, the ammonia removal rate is within ~20% to 40% (Cho et al., 2009; Ye et al., 2011), and the phosphorus removal rate is relatively high, ranging from 67% to a maximum of 99% (Kim et al., 2004; Liu et al., 2011a).
Observation of struvite crystallization
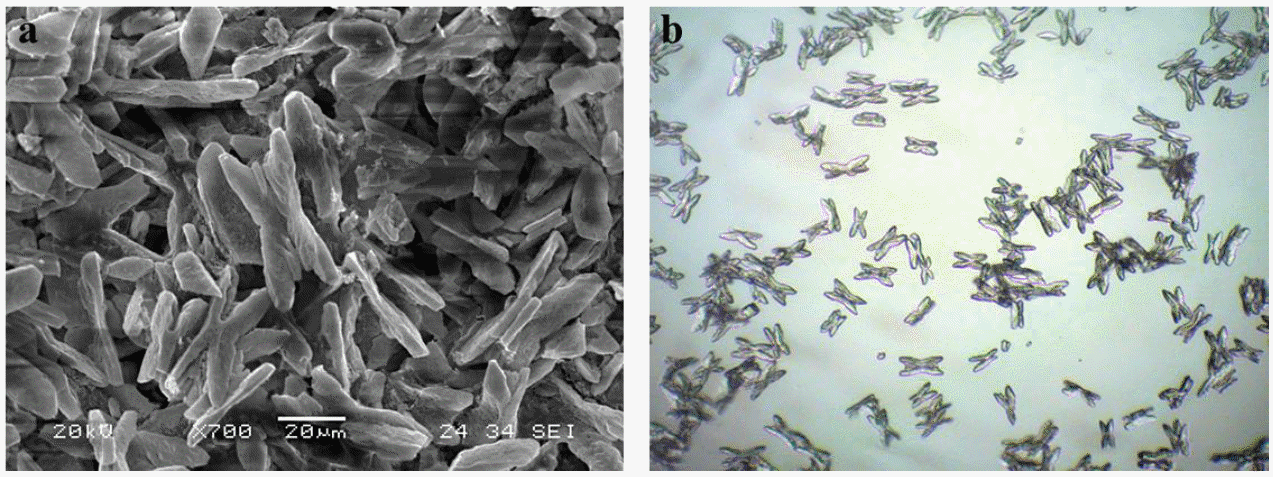
Figure 4 shows the microscopic picture of the struvite crystal generated by adding the MgCl2·6H2O. Figure 4a was the scanning electron microscope image of the MAP (magnesium ammonium phosphate) crystal generated by mixing the MgCl2·6H2O for 5 days at 100 rpm and pH 8.8. The morphology was X-shaped branch crystal and the size was about 45 to 55 μm. Figure 4b was the light microscope image with a magnification of 100 times.
It has been reported that the MAP crystal generated in livestock wastewater tends to be treated repetitively and increases in size as time passes. Ueno and Fujii (2001) reported that the size of a struvite crystal grows by 0.5 to 1 mm during a 10 day-long reaction, and Schulze-Rettmer et al. (2001) reported that a crystal with a size of 1 cm was found in their manure storage tank. Published morphology types range from coffin-like (Wierzbicki et al., 1997) and needle-like (Abbona and Boistelle, 1985) to trapezoidal (Munch and Barr, 2001). All of these forms are described as ‘typical for struvite’. In our experiment, X-shaped branched crystals were observed. This shape was reported by Mariska et al. (2010).
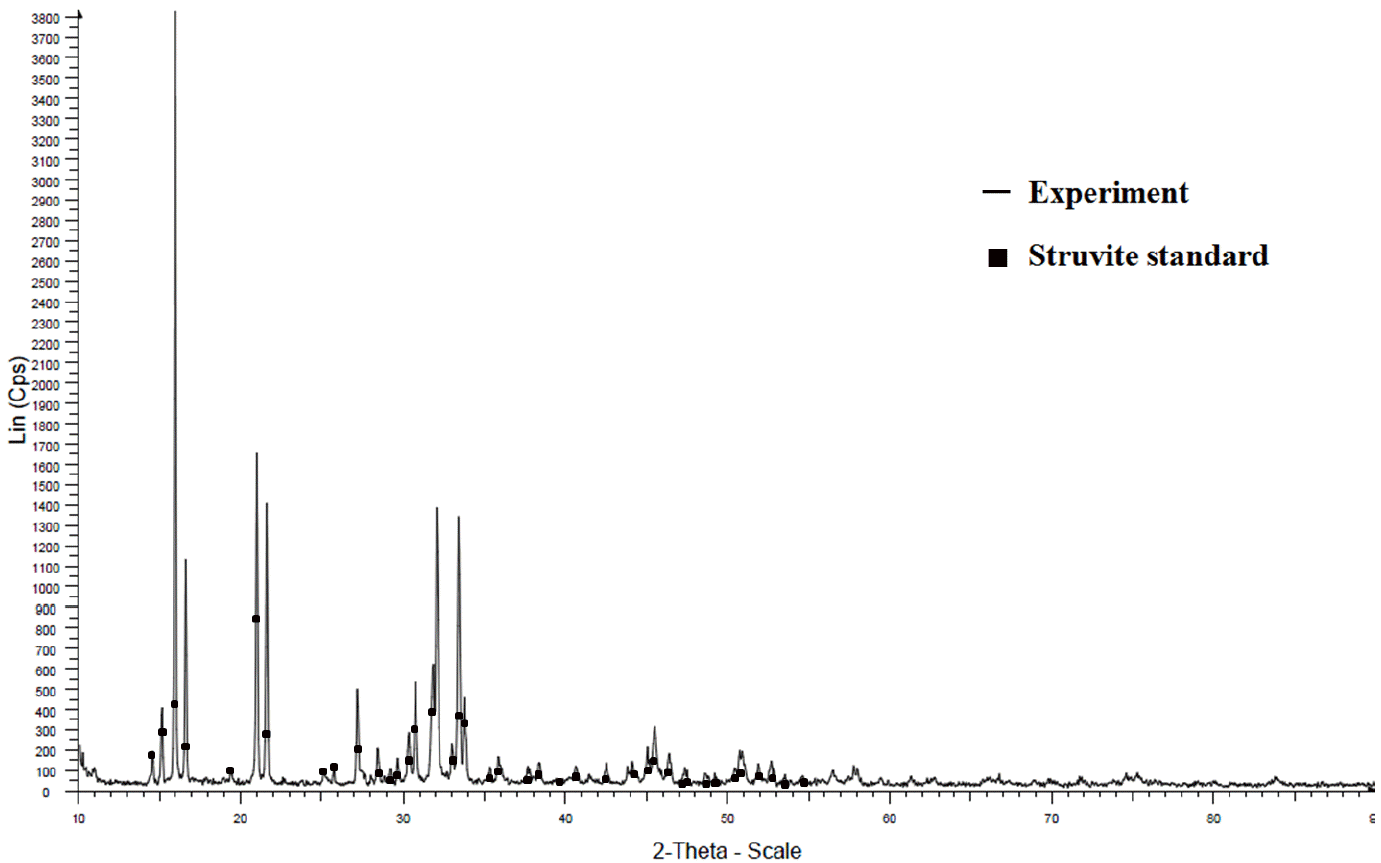
The solids obtained in the experiments were subjected to XRD analysis. The powdered XRD pattern of synthetic and struvite pellets matched very well with that of the published pattern for struvite (Figure 5). The slight difference between the XRD patterns of the two kinds of struvite may be due to the amount of impurity present in the struvite pellets. Infrared (IR) spectra for both synthetic struvite and struvite pellets were also consistent with the published IR spectrum of struvite in the wave number range of 400 to 4,000 cm−1 with 100% recovery of spectra according to the standards. As such, the struvite samples were considered to be a single-phase material.
CONCLUSION
Struvite crystallization using the anaerobic digestive fluid of swine manure containing highly concentrated nitrogen is a highly effective and eco-friendly process that can collect or remove phosphorus and nitrogen. If the potential hazardous substances in wastewater are not removed before being placed in an ecosystem, it can result in eutrophication. However, if they are collected, they can be precious resources. The effect of pH, temperature, reaction mixing speed, and the Mg2+:PO43− ratios, which are important factors that affect the struvite crystallization reaction, was determined. In this study, approximately 88% of P and 40% of N were removed and collected from the existing livestock wastewater through struvite crystallization. However, even if the N removal efficiency is low, a tremendous amount of nitrogen in the wastewater can be removed through ammonia stripping. Struvite is an important material that can become a major source of slow-release fertilizers using phosphorus even after the phosphorus in the phosphorus mines is completely exhausted.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print







