 |
 |
Abstract
A 15-wk feeding trial was conducted to examine the supplemental effects of Barodon on growth performance, gastrointestinal histology, feed digestibility and innate immunity in olive founder. A basal commercial diet was used as a control and two other diets were prepared by spraying 0.1% or 0.2% of Barodon. Triplicate groups of fish (BW, 145 g) were fed one of the test diets to apparent satiation twice daily. At the end of the feeding trial, fish growth performance was not significantly affected by dietary treatments; however, feed utilization was significantly improved (linear and quadratic, p<0.05) by Barodon supplementation. Significantly higher (p<0.05) survival rates were obtained in fish fed Barodon containing diets. Hepatosomatic index increased significantly in Barodon treated groups. Also, the use of Barodon resulted in significant increase (linear and quadratic, p<0.05) of intestine length and number of goblet cells. Significantly higher (Quadratic, p<0.05) apparent digestibility coefficient of DM was obtained by supplementation of Barodon. Lysozyme and myeloperoxidase activities increased quadratically and linearly, respectively, in Barodon treated fish. Also, significantly higher (linear and quadratic, p<0.05) superoxide dismutase activity was found in Barodon fed fish. The findings in this study show that inclusion of Barodon in diets for olive flounder improves feed utilization and digestibility, and positively affects digestive tract histology and innate immunity.
Aquaculture remains one of the fastest-growing animal food-producing sectors accounting for almost half of the total food fish production (FAO, 2010). Aquaculture has expanded during the past several decades, and this industry has a high density stocking strategy due to the pursuit of maximum productivity in limited culture systems. Intensive aquaculture triggers higher susceptibility to disease and significant losses in aquaculture. Protection of farmed fish from various diseases is a prerequisite for increasing production and further development of aquaculture. A wide range of antimicrobial compounds have been used for treatment of infectious bacterial diseases in aquaculture farms (Carnevail et al., 2004; Lauzon et al., 2010). Antibiotics have several harmful side effects, such as development of antibiotic resistant strains of pathogens and concerns regarding the antibiotic residues in the environment and food safety (Kumari and Sahoo, 2006; Sahu et al., 2007). Therefore, a prerequisite for aquaculture to prosper in the future is to minimize the use of antibiotics in controlling fish disease (Wu et al., 2007).
Prevention of fish disease through stimulation of the immune system is considered as a promising approach for sustainable aquaculture (Ardo et al., 2008). Immunostimulants enhance the overall resistance against pathogens by stimulation of the non-specific immune response (Sakai, 1999; Gannam and Schrock, 2001) which plays a key role in immunity during the initial stages of infection (Divyagnaneswari et al., 2007; Swain et al., 2007).
Several immunostimulants have been examined in fish and shrimp including both synthetic chemicals and natural biological substances (Sakai, 1999). Barodon, an anionic alkali mineral complex, has been identified as a potential immunstimulant in terrestrial animals, such as pigs and horses. It is composed of an alkali solution (pH 13.5) containing Si, Ag and K ions. Its immunomodulatory activity has been demonstrated by proliferation and activation of porcine immune cells, particularly CD4+CD8+ double-positive T lymphocytes in peripheral blood and in the secondary lymphoid organ (Yoo et al., 2001, 2002). Also, it was shown to have an adjuvant effect on hog cholera vaccine efficiency (Park et al., 2000). However, to the best of our knowledge there is no available information on its beneficial effects in fish.
Olive flounder is one of the most important fish species for marine aquaculture in Asian countries, and has been successfully cultured in Korea, Japan and China (Kang et al., 2008; Casta├▒o-S├Īnchez et al., 2010). Regarding the commercial importance of this species in Korea, a feeding trial was undertaken to examine the supplemental effects of Barodon on growth performance, feed utilization, non-specific immune response, gastrointestinal histology and feed digestibility.
A basal commercial diet (Cargill Agri Purina Co., South Korea) with 55% crude protein and 7% crude lipid was used as a control and two other diets were prepared by supplementation of 0.1% or 0.2% Barodon (Barodon-S.F, Korea). Composition of Barodon used in this study is shown in Table 1. The targeting concentrations of Barodon were sprayed to the basal diet after dilution in distilled water (2% of diet wt). The control diet was treated similarly but no Barodon was included. The diets were dried at room temperature using electric fans and stored at ŌłÆ20┬░C until use.
Growing olive flounders were transported from a private hatchery (Dong-won Fisheries, Jeju Island, Korea) to Marine and Environmental Research Institute, Jeju National University (Jeju, Korea). All fish were acclimated to the experimental conditions and facilities for 2 wk. At the end of the acclimation period, twenty fish averaging at 145 g were stocked in each polyvinyl circular tanks of 400 L capacity. The tanks were supplied with filtered seawater at a flow rate of 4 L/min and aeration to maintain enough dissolved oxygen. Triplicate groups of fish were fed one of the diets to apparent satiation twice a day (08:00 and 18:00 h) for 15 wk. Uneaten food was siphoned out after 30 min of feeding and weighed to determine feed intake. Growth of fish was measured at every 3 weeks. Feeding was stopped 24 h prior to weighing or blood sampling to minimize stress on the fish.
At the end of the feeding trial, all the fish in each tank were weighed, counted and their total and fork length were measured for calculation of growth parameters and survival. Also, three fish per tank (nine fish per dietary treatment) were randomly captured, anaesthetized with 2-phenoxyethanol solution (200 mg/L) and blood samples were taken from the caudal vein with heparinized syringes to determine respiratory burst activity and total immunoglobin level. Additionally, another set of blood samples were taken from the caudal vein of three fish from each tank using nonheparinized syringes, allowed to clot at room temperature for 30 min, and the serum was separated by centrifugation for 10 min at 5,000├Śg and stored at ŌłÆ70┬░C for analysis of the other innate immune response parameters including lysozyme, myeloperoxidase (MPO), superoxide dismutase (SOD) and antiprotease activities.
The oxidative radical production by phagocytes during respiratory burst was measured using the NBT (nitro-blue-tetrazolium) assay described by Anderson and Siwicki (1995). Briefly, blood and NBT (0.2%) (Sigma, St. Louis, MO, USA) were mixed in equal proportion (1:1) and incubated for 30 min at room temperature. Then, 50 ╬╝L was removed and dispensed into glass tubes. One mL of dimethylformamide (Sigma) was added and centrifuged at 2,000├Śg for 5 min. Finally, the optical density of the supernatant was measured at 540 nm using a spectrophotometer (Genesys 10UV, Rochester, NY, USA). Dimethylformamide was used as the blank.
A turbidometric assay was used to determine serum lysozyme activity using the method described by Hultmark (1980) with slight modifications. Briefly, Micrococcus lysodeikticus (0.75 mg/mL) was suspended in sodium phosphate buffer (pH 6.4, 0.1 M), 200 ╬╝L of suspension was placed in each well of a 96-well plate, and 20 ╬╝L of serum was added. The reduction in absorbance of samples was determined at 570 nm in a microplate reader (UVM 340, Biochrom, Cambridge, UK) after a room temperature incubation for 0 and 30 min. Hen egg white lysozyme (Sigma) was used for the standard curve. Values are expressed as ╬╝g/mL.
Serum MPO activity was measured according to Quade and Roth (1997). Briefly, 20 ╬╝L of serum was diluted with Hanks Balanced Salt Solution (HBSS, Sigma) without Ca2+ and Mg2+ in 96-well plates. Then, 35 ╬╝L of 3,3ŌĆ▓,5,5ŌĆ▓-tetramethylbenzidine hydrochloride (TMB, 20 mM) (Sigma) and H2O2 (5 mM) were added. The color change reaction was stopped after 2 min by adding 35 ╬╝L of 4 M sulfuric acid. Finally, optical density was read at 450 nm in a microplate reader.
SOD activity was measured by the percentage reaction inhibition rate of enzyme with water soluble tetrazolium dye substrate and xanthine oxidase using a SOD Assay Kit (Sigma, 19160) according to the manufacturerŌĆÖs instructions. Each endpoint assay was monitored by absorbance at 450 nm (the absorbance wavelength for the colored product of the WST-1 reaction with superoxide) after 20 min of reaction time at 37┬░C. Percent inhibition was normalized by mg protein and presented as SOD activity units.
Plasma total immunoglobulin (Ig) level was determined according to the method described by Siwicki and Anderson (1993). Briefly, plasma total protein content was measured using a micro protein determination method (C-690; Sigma), prior to and after precipitating down the immunoglobulin molecules, using a 12% solution of polyethylene glycol (Sigma). The difference in protein content represents the Ig content.
Serum antiprotease activity was measured according to the method described by Ellis (1990) with slight modifications (Magnad├│ttir et al., 1999). Briefly, 20 ╬╝L of serum was incubated with 20 ╬╝L of standard trypsin solution (Type II-S, from porcine pancreas, 5 mg/mL, Sigma) for 10 min at 22┬░C. Then, 200 ╬╝L of phosphate buffer (0.1 M, pH 7.0) and 250 ╬╝L azocasein (2%) (Sigma) were added and incubated for 1 h at 22┬░C. Five hundred microliters of 10% trichloro acetic acid was added and further incubated for 30 min at 22┬░C. The mixture was centrifuged at 6,000├Śg for 5 min, and 100 ╬╝L of the supernatant was transferred to the wells of a 96 well flat bottomed microplate containing 100 ╬╝L of 1 N NaOH. Optical density was read at 430 nm. Buffer replaced the serum for the 100% positive control, and buffer replaced both serum and trypsin for the negative control. The trypsin inhibition percentage was calculated using the following equation:
where A1 = control trypsin activity (without serum); A2 = trypsin activity remaining after adding serum.
Also, at the end of the experiment three fish per tank were randomly selected in the early morning, killed with an overdose of 2-phenoxyethanol and dissected for measurement of hepatosomatic index (HSI) and relative length of the gut (RLG). For analysis of goblet cells, the intestine samples were fixed in BouinŌĆÖs solution, dehydrated in a graded series of ethanol, embedded in paraffin and then cut into 5 ╬╝m cross-sections. The slides were stained with Alcian blue (AB) at pH 2.5 and periodic acid-Schiff (PAS) for observation of the mucus- secreting goblet cells. The slides were observed using a light microscope (Carl Zeiss, HBO 50) with image scope 2.3 (Image Line, Inc) software. Photographs were taken using a canon digital photomicrographic system.
To examine the effect of Barodon on feed digestibility, the commercial diet used in the feeding trial was ground, supplemented with 0.1% and 0.2% Barodon and after addition of 1.0% chromic oxide (Cr2O3) as an inert indicator (Sigma-Aldrich, St. Louis, USA) and 30% double distilled water, it was then extruded through a meat chopper machine (SMC-12, Kuposlice, Busan, Korea) set at 3 mm in diameter. The pellets were dried with electric fans at room temperature and maintained at ŌłÆ20┬░C until use. Twenty fish (122 g) were distributed into each 300 L capacity Guelph system (fecal collection system) tanks. The tanks were supplied with cartridge-filtered seawater at a flow rate of 1 L/min and aeration. Duplicate groups of fish were hand-fed one of the test diets to apparent satiation once daily at 18:00 h. One hour after feeding, the rearing tanks were brushed out to remove uneaten feed and fecal residues. On the next day, feces were collected from the fecal collection columns. The collected feces in the tube was separated from supernatant water using a disposable paper filter and stored at ŌłÆ40┬░C. Then the fecal samples were freeze-dried for 24 h and stored at ŌłÆ20┬░C until analysis.
Chromium oxide content of diet and feces samples was analyzed according to the method described by Divakaran et al. (2002) at the Fish Feed and Nutrition Laboratory, Jeju National University, Jeju, Korea. Briefly, a known wt (5 to 10 mg) of ash samples of either diet or feces containing chromium oxide was placed in glass test tubes. Four milliliter of perchloric reagent was added along the sides of the test tube to wash down any adhering ash. Perchloric reagent was prepared as follows: two hundred milliliter of concentrated nitric acid was added to 100 mL of distilled water cooled down then 200 mL perchloric acid (70%) was added. The test tubes were set in a heating block and heated at 300┬░C for 20 min, for oxidation of chromium oxide to monochromate (CrO42-). Then the tubes were cooled down to room temperature and their contents were quantitatively transferred and made up to 25 mL in a volumetric flask by rinsing repeatedly with distilled water. The absorbance of samples was read at 350 nm using a spectrophotometer (Beckman DU-730, USA). A known wt (2 to 4 mg) of chromium oxide was similarly treated and used as standard.
The apparent digestibility coefficients (ADC) for DM and protein of the experimental diets were calculated through the following formulas:
All the diets were assigned by a completely randomized design. Data were analyzed using linear and quadratic orthogonal polynomial contrasts in SPSS version 19 (SPSS Inc., Chicago, IL, USA). Statistical significance was determined at 5% (pŌēż0.05). Percentage data were arcsine transformed before analysis.
The results of fish growth performance fed the experimental diets are presented in Table 2. Inclusion of Barodon in the diets resulted in a numerical increase of fish growth; but the differences were not significant (p>0.05). However, the use of Barodon significantly improved (linear and quadratic, p<0.05) feed utilization efficiency where a significantly lower feed conversion ratio and a higher protein efficiency ratio were found in Barodon treated groups. Also, significantly higher survival rates (p<0.05) were obtained in fish offered Barodon containing diets.
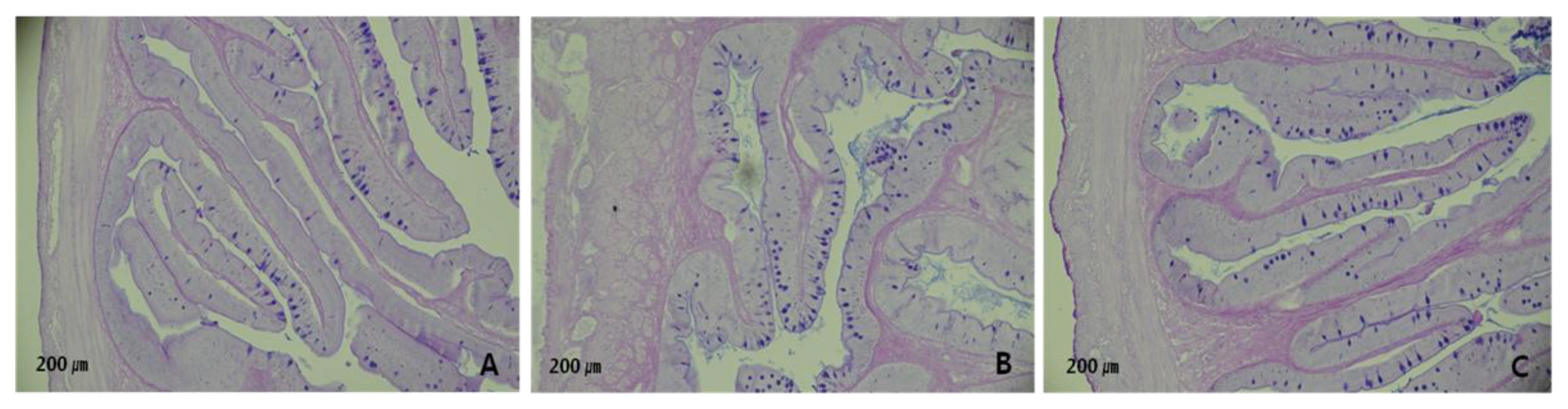
Dietary supplementation of Barodon resulted in the significant increase of hepatosomatic index and intestine length (linear and quadratic, p<0.05) (Table 2). The number of goblet cells was increased linearly and quadratically (p< 0.05) by Barodon supplementation (Table 2 and Figure 1).
Apparent digestibility coefficients for DM and protein of the experimental diets are provided in Table 3. Significantly higher (Quadratic, p<0.05) ADC of DM was found in Barodon fed fish and ADC of protein increased numerically by Barodon supplementation.
Significantly higher lysozyme (Quadratic, p<0.05) and MPO (linear, p<0.05) activities were found in Barodon fed fish (Table 4). Also, SOD activity significantly increased (linear and quadratic, p<0.05) in fish offered Barodon. However, NBT and antiprotease activities as well as Ig level were not significantly influenced (Table 4).
Results of this study showed the slight improvement of fish growth performance and significant increase of feed utilization efficiency by dietary supplementation of Barodon. There is limited available information on beneficial effects of Barodon in fish species. Barodon is a mixture of sodium silicate and certain metal salts in an alkaline solution, of which sodium silicate represents 60% of the total amount. The exact underlying mechanism of action of Barodon is unknown. However, it has been suggested that sodium silicate, the main mineral ingredient, decomposes quantitatively into bioavailable ortho-silicic acid in the acidic gastric juice and is absorbed in the body (Jurki─ć et al., 2013). Carlisle (1969, 1970) reported that silicon is uniquely localized in active growth areas in bones of animals. It has been shown that silicon plays essential roles in growth and skeletal development in chicks. A significant reduction in skeletal development was observed when the chicks were deprived of silicon (Carlisle, 1970). Such biological effects of silicon have been attributed to its interrelationships with other elements in the body including molybdenum, aluminium and calcium (Smith, 1993; Exley et al., 2006).
Activity of digestive enzymes is greatly influenced by pH. Indeed, the digestion process is performed in a neutral-alkaline condition (Munilla-Mor├Īn and Saborido-Rey, 1996a). Munilla-Mor├Īn and Saborido-Rey (1996a,b) reported that the optimum pH range for the action of digestive protease and amylase enzymes are 9.5ŌĆō10 and 7.0ŌĆō7.5, respectively, in gilthead seabream (Sparus aurata). Also, Sabapathy and Teo (1995) found that the optimal chymotrypsin activity of rabbitfish occurs at a pH range from 7.5 to 8.0. The improvement of fish feed utilization in the present study can be at least partially due to the enhancement in digestive enzymes activity, as Barodon is an alkaline solution and can aid in better enzymatic digestion.
The results of digestibility test in this study provide more evidence for positive effects of Barodon supplementation on fish feed utilization. The results showed a significant increase in digestibility of DM by inclusion of 0.1% Barodon and slight improvement of dietary protein digestibility in Barodon treated groups. Such enhancement in feed digestibility can be probably due to the facilitated digestive enzymes activity, particularly proteolytic enzymes.
On the other hand, Barodon serves as a supplemental source of minerals. Minerals are used for maintenance of skeletal formation and colloidal system, acid-base equilibrium and are used for biologically important compounds like the hormones and enzymes (Watanabe et al., 1997). It has been shown that deficiency of minerals results in biochemical, structural and functional pathologies.
Digestive tissues are flexible and show morphological changes in response to dietary manipulations (Starck, 1999). It has been demonstrated that fasting, increases in feed intake and changes in diet composition affect gut morphological parameters (Naya et al., 2007; Olsson et al., 2007). The length of gut is one of the important factors in nutrient absorption (Hofer and Schiemer, 1981). Ricklefs (1996) and Lavin et al. (2008) reported that the changes in intestine length influence the digestion and assimilation of nutrients. In the current study, significant increases were found in intestine length in Barodon fed groups. Such increase in the intestine length can positively affect nutrient digestion and absorption by enhancement of surface area, volume, villus area and other involving features.
Histological studies provide an approach for understanding pathological changes related to dietary manipulations (Gargiulo et al., 1998). In the present study, a higher number of mucus-secreting goblet cells was found in the anterior intestine of Barodon treated groups. Goblet cells are distributed throughout the gastrointestinal tract and provide protection against damage from chemical substances and digestive enzymes by secreting mucus (Allen et al., 1986). Also, they are responsible for the production of high molecular wt glycoprotein compounds known as mucins which is the major component of the intestinal mucus layer (Kim and Khan, 2013). Mucins are the key protective component of mucus. Murray et al. (1996) reported that the increment of goblet cells number results in higher mucosal membrane protection. Moreover, Osman and Caceci (1991) and Domeneghini et al. (2005) suggested that goblet cells positively affect the absorption of digestible substances. There is now abundant evidence that the mucins are probably the first molecules that interact with pathogens and provide protection by inhibiting binding to other glycoproteins and neutralizing the pathogens. The enhancement of goblet cells number in the current study can result in improvements in feed utilization and absorption as well as higher protection against invading pathogens.
Immunostimulatory activity of Barodon has been earlier reported in horses (Koo et al., 2006) and pigs (Yoo et al., 2001). Its immunomodulatory activity has been reported in pigs to be through the proliferation and activation of porcine immune cells, particularly CD4+CD8+ double-positive T lymphocytes (Yoo et al., 2001, 2002). Jurki─ć et al. (2013) suggested that ortho-silicic acid is responsible for the pharmacological effects of Barodon. The exact mode of action of Barodon is not clear, but it has been assumed that its mineral components affect the membrane-associated lymphoid tissues (Koo et al., 2006). Innate immune response of olive flounder was upregulated by Barodon in this study, and significantly higher lysozyme, MPO and SOD activities were found by Barodon supplementation. However, the other tested immune parameters were not significantly influenced. The innate immune system of fish comprises several humoral and cellular factors that may differentially respond to the offered immunostimulant (Secombes, 1996); accordingly different results were obtained for the examined immune parameters in the current study.
In conclusion, the results showed the beneficial effects of dietary supplementation of Barodon on feed utilization, gut histology and innate immunity of olive flounder. However, further studies are necessary for establishment of the optimum Barodon level in diets for olive flounder.
ACKNOWLEDGEMENTS
This study was supported by Cargill Agri Purina, Inc. and Barodon-S.F. Corp. Salaries were partly supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011-0015925).
Figure┬Ā1
Microphotographs of mucus-secreting goblet cells in anterior intestine of olive flounder fed the experimental diets for 15 wk.
Table┬Ā1
Composition of major ingredients for Barodon
| Ingredient | Amount |
|---|---|
| Na2SiO3 (g) | 600 |
| K2CO3 (g) | 300 |
| Na2CO3 (g) | 9 |
| Na2B4O7 (g) | 9 |
| C12H22O11 | q.s.1 |
| AgNO3 | q.s. |
| NaCl | q.s. |
| Na2S2O3 (g) | 0.12 |
| H2O (mL) | 1,000 |
Table┬Ā2
Growth performance and organosomatic indices of olive flounder (BW, 145 g) fed the experimental diets for 15 wk1
| Items | Barodon concentration (%) | SEM | p value | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Control | 0.1 | 0.2 | Linear | Quadratic | ||
| FBW2 | 502 | 541 | 550 | 26.48 | 0.59 | 0.15 |
| WG3 | 246 | 271 | 275 | 19.35 | 0.10 | 0.21 |
| SGR4 | 0.91 | 1.01 | 1.02 | 0.08 | 0.11 | 0.21 |
| FCR5 | 1.25 | 1.01 | 0.91 | 0.06 | <0.05 | <0.05 |
| PER6 | 1.38 | 1.72 | 1.91 | 0.11 | <0.05 | <0.05 |
| Survival (%) | 70.7 | 92.0 | 96.0 | 5.81 | <0.05 | <0.05 |
| CF7 | 1.13 | 1.14 | 1.09 | 0.07 | 0.45 | 0.63 |
| HSI8 | 1.72 | 2.16 | 2.35 | 0.16 | <0.05 | <0.05 |
| RLG9 | 63.4 | 82.8 | 81.4 | 6.28 | <0.05 | <0.05 |
| Goblet cells number | 1,456 | 1,834 | 2,342 | 257 | <0.05 | <0.05 |
Table┬Ā3
Apparent digestibility coefficients (ADC, %) for DM and CP of the experimental diets for olive flounder1
| Items | Barodon concentration (%) | SEM | p value | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Control | 0.1 | 0.2 | Linear | Quadratic | ||
| DM | 77.2 | 79.8 | 76.7 | 0.74 | 0.71 | <0.05 |
| CP | 86.9 | 89.8 | 88.4 | 1.18 | 0.29 | 0.07 |
Table┬Ā4
Non-specific immune responses of olive flounder fed the experimental diets for 15 wk1
| Items | Barodon concentration (%) | SEM | p value | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Control | 0.1 | 0.2 | Linear | Quadratic | ||
| NBT2 | 0.84 | 0.96 | 0.88 | 0.12 | 0.71 | 0.47 |
| MPO3 | 1.29 | 1.58 | 1.88 | 0.23 | <0.05 | 0.06 |
| Lysozyme4 | 17.26 | 31.38 | 25.71 | 5.00 | 0.18 | <0.05 |
| Ig5 | 18.74 | 20.64 | 18.24 | 1.53 | 0.75 | 0.21 |
| Antiprotease6 | 16.10 | 17.37 | 20.52 | 2.64 | 0.07 | 0.19 |
| SOD7 | 69.92 | 74.59 | 82.22 | 3.78 | <0.05 | <0.05 |
REFERENCES
Allen A, Hutton DA, Leonard AJ, Pearson JP, Sellers LA. 1986. The role of mucus in the protection of the gastroduodenal mucosa. Scand J Gastroenterol 21:71ŌĆō78.

Anderson DP, Siwicki AK. 1995. Basic haematology and serology for fish health programs. Diseases in Asian aquaculture II. Shariff M, Arthur JR, Subasinghe RP, editorsFish health section. Asian Fisheries Society; Manila: p. 185ŌĆō202.
Ardo L, Yin G, Xu P, Varadi L, Szigeti G, Jeney Z, Jeney G. 2008. Chinese herbs Astragalus membranaceus and Lonicera japonica and boron enhance the non-specific immune response of Nile tilapia Oreochromis niloticus and resistance against Aeromonas hydrophila. Aquaculture 275:26ŌĆō33.

Carlisle EM. 1969. Silicon localization and calcification in developing bone. Fed Proc 28:374
Carlisle EM. 1970. A relationship between silicon and calcium in bone formation. Fed Proc 29:565
Carnevali O, Zamponi MC, Sulpizio R, Rollo A, Nardi M, Orpianesi C, Silvi S, Caggiano M, Polzonetti AM, Cresci A. 2004. Administration of probiotic strain to improve sea bream wellness during development. Aquacult Int 12:377ŌĆō386.

Casta├▒o-S├Īnchez C, Fuji K, Ozaki A, Hasegawa O, Sakamoto T, Morishima K, Nakayama I, Fujiwara A, Masaoka T, Okamoto H, Hayashida K, Tagami M, Kawai J, Hayashizaki Y, Okamoto N. 2010. A second generation genetic linkage map of Japanese flounder (Paralichthys olivaceus). BMC Genomics 11:554



Divakaran S, Leonard GO, Ian PE. 2002. Note on the methods for determination of chromic oxide in shrimp feeds. J Agric Food Chem 50:464ŌĆō467.


Divyagnaneswari M, Christybapita D, Michael RD. 2007. Enhancement of nonspecific immunity and disease resistance in Oreochromis mossambicus by Solanum trilobatum leaf fractions. Fish Shell Immunol 23:249ŌĆō259.

Domeneghini C, Arrighi S, Radaelli G, Bosi G, Veggetti A. 2005. Histochemical analysis of glycoconjugate secretion in the alimentary canal of Anguilla anguilla L. Acta Histochemica 106:477ŌĆō487.


Ellis AE. 1990. Serum antiprotease in fish. Techniques in Fish Immunology. Stolen JS, Fletcher TC, Anderson DP, Roberson BS, Van Muiswinker WB, editorsSOS Publication; Fair Haven, New Jersey, USA: p. 95ŌĆō99.
Exley C, Korchazhkina O, Job D, Strekopytov S, Polwartand A, Crome P. 2006. Noninvasive therapy to reduce the body burden of aluminium in AlzheimerŌĆÖs disease. J Alzheimers Dis 10:17ŌĆō24.


FAO. 2010. The State of World Fisheries and Aquaculture 2010. Food and Agriculture Organization of the United Nations; Rome:
Gannam AL, Schrock RM. 2001. Immunostimulants in fish diets. Nutrition and Fish Health. Lim C, Webster CD, editorsFood Products Press; New York, USA: p. 235ŌĆō266.

Gargiulo AM, Ceccarelli P, DallŌĆÖAglio C, Pedini V. 1998. Histology and ultrasturucture of the gut of the tilapia (Tilapia spp.), a hybrid teleost. Anat Histol Embryol 27:89ŌĆō94.


Hofer R, Schiemer F. 1981. Proteolytic activity in the digestive tract of several species of fish with different feeding habits. Oecologia 48:342ŌĆō345.


Hultmark D. 1980. Insert immunity: purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem 106:7ŌĆō16.


Jurki─ć LM, Cepanec L, Paveli─ć SK, Paveli─ć K. 2013. Biological and therapeutic effects of ortho-silicic acid and some ortho-silicic acid releasing compounds: new perspectives for therapy. Nutr Metab 10:2



Kang JH, Kim WJ, Lee WJ. 2008. Genetic linkage map of olive flounder, paralichthys olivaceus. Int J Biol Sci 4:143ŌĆō149.



Kim JJ, Khan WI. 2013. Goblet cells and mucins: Role in innate defense in enteric infections. Pathogens 2:55ŌĆō70.



Koo HC, Ryu SH, Ahn HJ, Jung WK, Park YK, Kwon NH, Kim SH, Kim JM, Yoo BW, Choi SI. 2006. Iuunostimulatory effects of the anionic alkali mineral complex Barodon on Equine lymphocyte. Clin Vaccine Immunol 13:1255ŌĆō1266.



Kumari J, Sahoo PK. 2006. Dietary levamisole modulates the immune response and disease resistance of Asian catfish Clarias batrachus(Linnaeus). Aquacult Res 37:500ŌĆō509.

Lauzon HL, Margeirsson B, Sveinsd├│ttir K, Gudj├│nsd├│ttir M, Karlsd├│ttir MG, Martinsd├│ttir E. 2010. Overview on fish quality research: impact of fish handling, processing, storage and logistics on fish quality deterioration. Sk├Įrsla Mat├Łs 39-10. p. 70
Lavin SR, Karasov WH, Ives AR, Middleton KM, Garland T. 2008. Morphometrics of the avian small intestine, compared with non-flying mammals: a phylogenetic approach. Physiol Biochem Zool 81:526ŌĆō550.


Magnad├│ttir B, Jonsdottir H, Helgason S, Bjornsson B, J├Ėrgensen T, Pilstr├Č L. 1999. Humoral immune parameters in Atlantic cod (Gadus morhua L) I: the effects of environmental temperature. Comp Biochem Physiol Part B Biochem Mol Biol 122:173ŌĆō180.

Munilla-Mor├Īn R, Saborido-Rey F. 1996a. Digestive enzymes in marine species. I. Proteinase activities in gut from redfish (Sebastes mentella), seabream (Sparus aurata) and turbot (Scophthalmus maximus). Comp Biochem Physiol Part B Biochem Mol Biol 113:395ŌĆō402.

Munilla-Mor├Īn R, Saborido-Rey F. 1996b. Digestive enzymes in marine species. II. Amylase activities in gut from seabream (Sparus aurata), turbot (Scophthalmus maximus) and redfish (Sebastes mentella). Comp Biochem Physiol Part B Biochem Mol Biol 113:827ŌĆō834.

Murray HM, Wright GM, Goff GP. 1996. A comparative histological and histochemical study of the post gastric alimentary canal from three species of pleuronectids, the Atlantic halibut, the yellowtail flounder and the winter flounder. J Fish Biol 48:187ŌĆō206.

Naya DE, Karasov WH, Bozinovic F. 2007. Phenotypic plasticity in laboratory mice and rats: Meta-analysis of current ideas on gut size flexibility. Evol Ecol Res 9:1363ŌĆō1374.
Olsson J, Quevedo M, Colson C, Svanback R. 2007. Gut length plasticity in perch: into the bowels of resource polymorphisms. Biol J Linn Soc 90:517ŌĆō523.

Osman AHK, Caceci T. 1991. Histology of the stomach of Tilapia nilotica(Linnaeus, 1758) from the River Nile. J Fish Biol 38:211ŌĆō223.

Park BK, Park YH, Seo KS. 2000. Relation between lymphocyte subpopulations of peripheral blood and immune responses of modified live hog cholera virus vaccine in pigs treated with an ionized alkali mineral complex. J Vet Sci 1:49ŌĆō52.


Quade MJ, Roth JA. 1997. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet Immunol Immunopathol 58:239ŌĆō248.


Ricklefs RE. 1996. Morphometry of the digestive tracts of some passerine birds. The Condor 98:279ŌĆō292.

Sabapathy U, Teo LH. 1995. Some properties of the intestinal proteases of the rabbitfish, Siganus canaliculatus(Park). Fish Physiol Biochem 14:215ŌĆō221.


Sahu S, Das BK, Mishra BK, Pradhan J, Sarangi N. 2007. Effect of Allium sativum on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. J Appl Ichthyol 23:80ŌĆō86.

Secombes CJ. 1996. The nonspecific immune system: cellular defenses. The fish immune system Organism, pathogen and environment. Iwama G, Nakanishi T, editorsAcademic press; San Diego, CA, USA: p. 63ŌĆō101.

Siwicki AK, Anderson DP. 1993. Nonspecific defense mechanisms Assay in fish. II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin level in serum. Siwicki AK, Anderson DP, Waluga J, editorsFish Disease Diagnosis and Prevention Methods; Olsztyn, Poland: p. 105ŌĆō112.
Smith BL. 1993. Analysis of hair element levels by age, sex, race, and hair color. In trace elements in man and animals, TEMA 8. Anke M, Meissner D, Mills CF, editorsKluwer; New York, USA: p. 1091ŌĆō1093.
Starck JM. 1999. Structural flexibility of the gastro-intestinal tract of vertebrates: implications for evolutionary morphology. Zool Anz 238:87ŌĆō101.
Swain P, Dash S, Sahoo PK, Routray P, Sahoo SK, Gupta SD, Meher PK, Sarangi N. 2007. Non-specific immune parameters of brood Indian major carp Labeo rohita and their seasonal variations. Fish Shell Immunol 22:38ŌĆō43.

Wu G, Yuan C, Shen M, Tang J, Gong Y, Li D, Sun F, Huang C, Han X. 2007. Immunological and biochemical parameters in carp Cyprinus carpio after Qompsell feed ingredients for long-term administration. Aquacult Res 38:246ŌĆō255.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





