 |
 |
Abstract
The aim of this study was to compare both the performance of litters derived from two sire genetic origins (SGO), Vienna Blue (VB) and Burgundy Fawn (BF), along successive seasons of birth (SB; winter, spring, summer and autumn), and doe reproductive performance in an organic production system. A total of fifty-eight does consisting of a mixture of crosses of several medium-large size breeds at different parity order (P, 1 = nulliparous; 2 = primiparous; âĽ3 = multiparous) and twelve males (6 VB and 6 BF) were housed indoors at environmental conditions that followed seasonality. An extensive reproductive rhythm was used and kits were weaned at 46Âą6 d of age. Doe reproductive performance and the data of 105 litters (55 from VB and 50 from BF SGO) were recorded throughout the SB. No statistically significant differences related to SGO effect were observed. As regards parity order, multiparous does showed higher live weights (LW) (p<0.05), total born (p<0.01), total born alive (p<0.05) per delivery, and litter weight of born alive (p<0.05), but lower milk output at 21st d than primiparous does (p<0.05). The extensive reproductive rhythm mainly increased litter performance at birth in multiparous does but was not sufficient to permit a complete recovery of body reserves lost during lactation. Autumn SB negatively affected doe LW variation between deliveries. The number of pups born and born alive per delivery (p<0.05) and litter size at 21 d of age and at weaning (p<0.01) were lower during hot SB. Due to the lower litter size of pups born in summer and autumn, their individual weight at 21st d of age and daily individual growth rate 0 to 21 d were higher than those of pups born in winter (p<0.001). Litter performance at 21st d of age and individual pup pre-weaning growth rate were poorer for those born in spring than in other seasons due to the harmful effects of increased environmental temperatures. SB affected most of the performance traits of does and young rabbits reared under the organic farming system. The rabbits seemed better suited to organic rearing conditions during winter than in other seasons. The worst results overall were obtained in the spring SB, whereas the hot SB negatively affected both doe energy balance and prolificacy. In conclusion, the pups of the 2 SGO showed good pre-weaning performance and seemed suited to the organic rabbit production system.
Organic rabbit production in Italy must follow the guidelines for organic livestock systems (Council Regulation (EC) No. 1804/1999) and those of two official certification organisms (ICEA, 2007; AIAB, 2012). Only pure breeds, their first generation crosses, and local population are admitted, and red-eye breeds are forbidden. The presence of a large number of animal biodiversity is the basis for the extensive production system, and for this reason it is important to maintain pure breeds, crossed breeds, and local populations that are well suited to pre-existing environmental conditions. Furthermore, the choice of the breed must primarily consider resistance to disease and slow growth, even if reproductive traits and litter performance must be also taken into account. Over the last decade, several authors have studied the effects of alternative and organic rearing systems on the productive live performance, carcass and meat quality of rabbits derived from hybrids, pure breeds or local populations, but only a few have focused on reproductive performance (Cavani et al., 2000, 2004; Dal Bosco et al., 2000, 2002, 2003; Paci et al., 2004, 2005; Pla, 2008; DâAgata et al., 2009; Dalle Zotte et al., 2009; Princz et al., 2009). Many scientific papers and reviews report the effect of season and parity order on reproductive performances of does and productive performances of litters derived from hybrid lines reared under intensive system (Rommers et al., 1999; Marai et al., 2002; Xiccato et al., 2004; Fortun-Lamothe, 2006; Rebollar et al., 2009; Castellini et al., 2010; Villalobos et al., 2010; SzendorĹ et al., 2012), whereas these effects on purebred rabbit does, unselected for high production, and extensively reared are scarce (Paci et al., 2003). The information concerning the effect of the sire genetic origin on litter traits of synthetic lines, native and coloured breeds are also limited (Ozimba and Lukefahr, 1991; Khalil and Al-Saef, 2008; Piles et al., 2008a, b; SzendrĹ et al., 2010, 2012).
The aim of this study was to compare the performance of litters derived from two sire genetic origins, Vienna Blue and Burgundy Fawn, along successive rearing seasons, and doe reproductive performance in an organic production system.
The trial was carried out in a farm certified for organic production located in Lombardia region, Italy (Azienda Agricola âNoi e la Naturaâ, www.noielanatura.it) in 2004 from January to November.
A total of fifty-eight rabbit does at different parity order (P; 1 = nulliparous; 2 = primiparous; âĽ3 = multiparous) and twelve males were involved. The maternal genetic origin was a mixture of crosses of several medium-large size breeds, California and New Zealand White excluded, according to an Italian official certification organism âAssociazione Italiana per lâAgricoltura Biologicaâ (AIAB). The sire genetic origins (SGO) were Vienna Blue (VB; n = 6) and Burgundy Fawn (BF; n = 6) introduced from an outside source at 8 weeks of age.
Does and sires were housed indoor in the same building supplied with a forced-ventilation system and natural lighting. During the experiment, environmental conditions followed seasonality with temperatures ranging from 10°C to 30°C and relative humidity from 60 to 75%. The does were housed individually in wire cages (810Ă60Ă33 cm) with plastic slat floor according to the directives of EC Regulation 1804/1999 and the AIAB (2012). External nest boxes, containing a 2 cm layer of sanitized wood shavings, were provided at 28 d of gestation for kindling and nursing the kits up to weaning. Litters were equalised at birth by cross-fostering (8 to 10 pups per litter).
An organic pelleted diet was given ad libitum to rabbit does and litters during the experimental period. Males were first mated at 5 months of age; nulliparous females were first mated at 3.5 months of age through natural mating. The does were randomly halved and the two groups were assigned to one of the two sire lines for the duration of the experiment.
Does were re-offered to the male 31 to 36 d after each delivery and pregnancy diagnosis through abdominal palpation took place 15 d after each mating. Does diagnosed not pregnant were returned to another buck of the designated sire line for re-mating. The females were re-mated three times until diagnosed as pregnant, after the third negative mating, the females were culled. Along the trial 14 does mated with BF (5 at first, 7 at second, 1 at third and 1 at fourth kindling) and 12 does mated with VB (7 at first, 4 at second and 1 at third kindling) were culled and replaced by other does not previously mated with the males object of study.
The kits, born from January to November, were weaned at 46Âą6 d of age but weaning occurred in two steps: at 35 d of age, the litters were moved to a collective cage in front of the mother that maintained the litter unit before being moved again to the collective cages located in the fattening building. The free suckling system was applied; nest boxes were inspected every morning and dead rabbits were removed.
Doe reproductive performance and the data of 105 litters (55 litters from VB and 50 litters from BF SGO) were recorded throughout the seasons of birth (SB) (winter, spring, summer and autumn). Doe live weight was measured after each delivery. Day of delivery, number of total born, and born-alive per delivery, litter weight of born alive, litter size and weight at 21 d of age, pre-weaning mortality, weaning age, litter size, and weight at weaning were also recorded.
The following data were then calculated: fertility rate (percentage of deliveries/mating), delivery interval and doe LW variation between deliveries, milk output at 21st d of lactation (difference in litter body weight measured before and after suckling at 21 d of age), individual weight of born alive, at 21 d of age, and at weaning, and daily individual growth rate in ranges of 0 to 21 d, 22 d-weaning, and pre-weaning (0-weaning).
Throughout the trial, the organic diet was sampled for chemical analysis four times at each batch change (Table 1). AOAC procedures (2000) were used to determine in duplicate dry matter (DM; 934.01), ash (942.05), crude protein (CP; 976.05), crude fibre (CF; 978.10), neutral detergent fibre (NDF; 2,000.04), acid detergent fibre (ADF), and acid detergent lignin (ADL; 973.18) by using the Ankom Technology (Macedon, NY 14502) with filter bags (F57). Ether extract (EE; 920.39) was determined after acid-hydrolysis. Gross energy (GE) was determined by adiabatic bomb calorimeter (ISO, 1998).
ANOVA was performed using the SAS (2007) program by including the SGO (VB, BF) the season of birth (SB), the parity (P), and their interactions, for fertility rate only SGO effect was considered. No statistically significant interactions were observed for the considered parameters with exception of SGOxP interaction that resulted statistically significant (p<0.05) only for the variable âindividual weight of born aliveâ.
The statistical significance of the differences was assessed with the Tukey test (SAS, 2007).
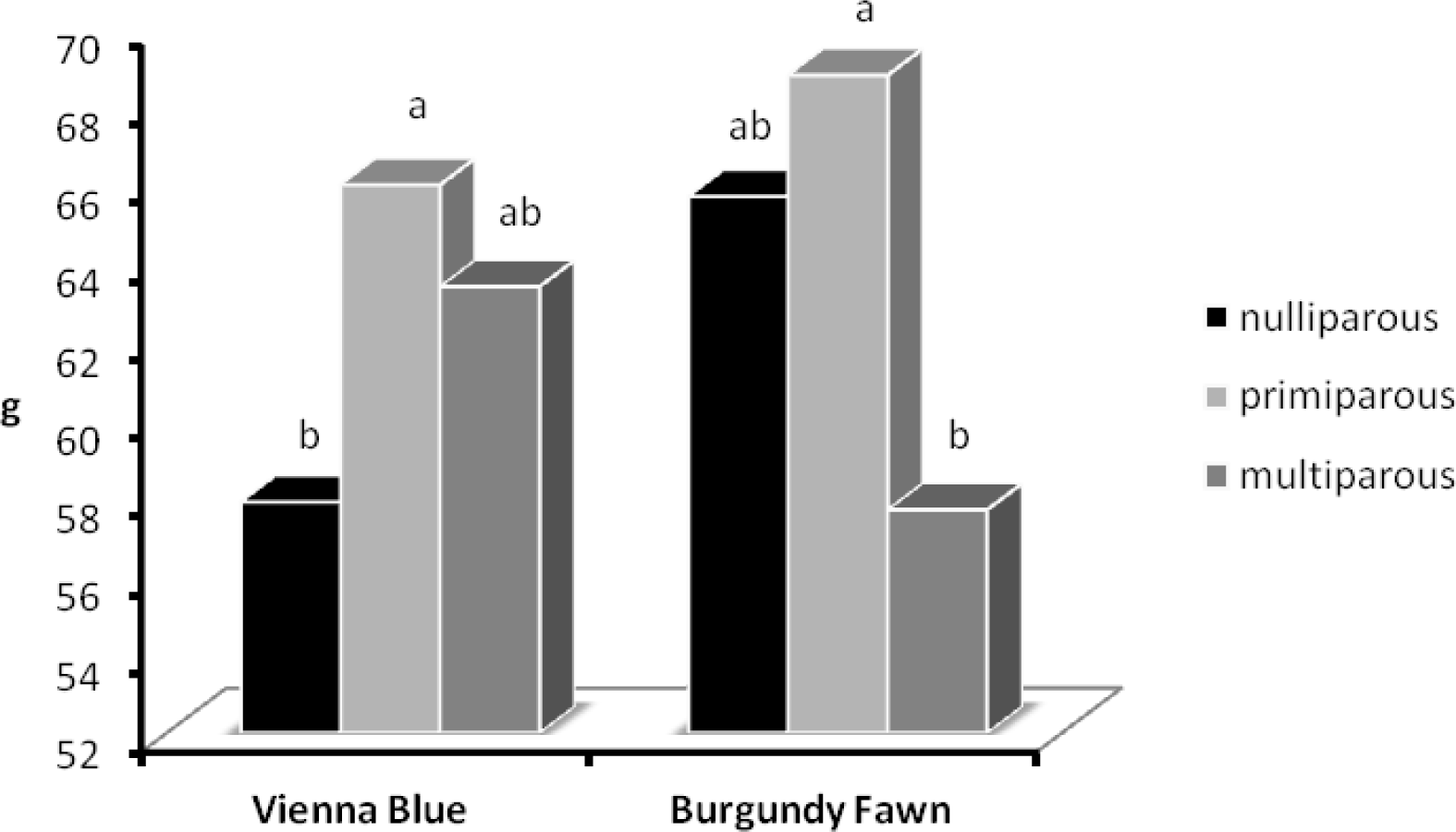
Interaction among main factors was not significant with exception for the SGOxP interaction (p<0.05) for individual weight of born alive pups (Figure 1) and thus Tables do not report the significance of the interaction.
Table 2, 3 and 4 summarize the effects of sire genetic origin (SGO), season of birth (SB), and parity order (P) on doe reproductive performance and on litter size and litter weight at birth, at 21 d, and at weaning, and throughout the 10 months of monitoring.
No significant differences related to the SGO effect were observed for all parameters.
The SB affected almost all the studied parameters. Considering doe LW variations between deliveries (Table 2), a significant LW loss in autumn SB was observed, whereas in winter and spring SB the doe energy balance was positive (â8.18 vs 2.75 and 0.22%, respectively; p<0.05). The slight doe LW loss during summer SB might be explained by the reduced appetite and consequent lower feed intake at moderately high temperatures that induces does to mobilize their body reserves to satisfy their production needs and leads to lower live body weight at kindling (Nardone et al., 2010). The higher doe LW loss during autumn SB could depend on the inability to recover the negative effect of heat suffered during the previous season combined with the energy mobilization required for greater milk production.
Litter size at birth was also affected by SB. The numbers of pups born/delivery and born alive/delivery were higher in winter than in autumn and summer SB (9.5 vs 7.5 and 7.8 pups; 9.4 vs 7.5 and 7.8 pups, respectively; p<0.05, Table 2); similar results were obtained also when considering litter size adjusted by cross-fostering (9.6 vs 7.9 and 7.9 pups; p<0.01, Table 2). The higher litter size constituted by cross-fostering, mainly in winter SB, depended on the decision of the breeder to keep all newborn and equalize the litters to the maximum in order to cover production costs. The SB effect on litter size at birth observed in this study confirms those reported elsewhere (Ayyat and Marai, 1998; Marai et al., 2002; Villalobos et al., 2010; SzendrĹ et al., 2012). The decrease in prolificacy at high environmental temperature depends on a great number of physiological events that may lead to significant reductions in total born and an increase in still-born pups. High environmental temperature has also been shown to compromise oocyte growth and embryo development while increasing embryo mortality (Wolfenson et al., 2000; Marai et al., 2006; Hansen, 2007). The reduced prolificacy observed in autumn may also depend on the effect of the heat stress suffered by the does during the previous hot season. High environmental temperatures are known to lead to reductions in feed intake that impair doe performance and alter the physiological functions that affect reproductive efficiency as a result (Lebas et al., 1986; Lacetera et al., 2003; Fortun-Lamothe, 2006).
SB also affected the individual weight of born alive pups: born alive pups born in winter SB were significantly lighter than those born in autumn SB (58.1 vs 69.1 g; p<0.05, Table 2), probably due to differences in litter size. Birth weight is influenced by several factors during pregnancy, among which, the number of fetuses in the uterine horn plays an important role (Rommers et al., 1999).
Parity order (P) influenced some of the variables considered (Table 2). The LW of multiparous does was significantly higher than that of nulliparous does (4,352 vs 4,122 g; p<0.05). The lighter LW observed in the nulliparous does is related to age: when does start their reproductive life, they have not yet usually achieved total body development (Rebollar et al., 2009). However, the values of LW variation between kindlings indicate an increase of the energy deficit with P despite an increase in doe LW or any extensive reproductive rhythm adopted. Thus, the extensive reproductive rhythm used in accordance with organic farming guidelines does not appear efficient in reducing doe energy deficits, even regardless of parity order. In these females, not selected for high productive and reproductive performances, the after weaning mating was not sufficient to permit a complete recovery of the body reserves lost during lactation. These findings were observed also by other authors in multiparous hybrids, which were unable to cover the requirements for reproduction without mobilizing body energy (Pascual et al., 2000).
The milk output registered at 21st d of lactation (Table 2) was lower in nulliparous and multiparous than in primiparous does (226 and 224 vs 274 g/d; p<0.05). The low milk production of nulliparous does depended on their insufficient feed intake, which led to the consequent inability to cover lactation requirements (Xiccato et al., 2004; Fortun-Lamothe, 2006). The low milk output of multiparous does may also have been related to their body energy deficit, as denoted by the loss of weight and the higher litter size at birth. These results contrast with those reported by Xiccato et al. (2004), who indicated an increase of milk production with increasing P and litter size. It should be noted that the data reported in literature refer to hybrid does, which are selected for very high feed intake, whereas the does used in our research were unselected and for this reason unable to adjust their voluntary feed intake to their energy requirements (Pascual et al., 2000).
As concerns litter performance at birth (Table 2), multiparous doe litters were numerically larger in terms of both total born/delivery (p<0.01) and born alive/delivery (p<0.05), and also heavier (p<0.05) than those of nulliparous and primiparous does (9.4 vs 8.0 and 7.8 pups; 9.1 vs 8.0 and 7.8 pups; 546 vs 479 and 478 g, respectively). In multiparous does, the higher litter weight of born alive was due to the higher number of born alive at kindling and not to pup individual weight, as the latter did not differ significantly among P. Our findings are supported by other recent studies that considered multiparous females inseminated after weaning (Fortun-Lamothe, 2006; Theau-Clement, 2007; Castellini et al., 2010). In our study, does were mated with extensive reproductive rhythm, and therefore the negative effect of lactation on prolificacy was lacking and this led to improved litter parameters.
Data showing the statistically significant (p<0.05) SGOxP interaction for individual weight of born alive pups are reported in Figure 1. In VB SGO, the individual weight of born alive pups slightly increased, whereas in BF SGO it decreased significantly (p<0.05) with P. This individual birth weight decrease (from 68.8 to 57.7 g, in primiparous and multiparous BF, respectively) was probably related to the very high individual weight of pups of part of the does formerly primiparous.
Litter size, litter weight, individual weight, and daily individual growth rate were affected by SB also at 21 d of age (Table 3). In our study, the litter size at 21 d of age showed the lowest values in pups born in summer and autumn compared to those born in winter (6.9 and 7.5 vs 8.8 pups/L, respectively; p<0.01). These results might be explained by the lower number of born alive/delivery observed in summer and autumn and by the slightly higher pre-weaning mortality registered in summer.
In rabbits, the negative consequences of high environmental temperatures are also seen during autumn with the impairment of production and reproduction (Ayyat et al., 1995; Ayyat and Marai, 1998; Marai et al., 2002; Marai and Rashwan, 2004).
SB affected also individual weight at 21st d of age and the daily individual growth rate 0 to 21 d of age, creating heavier rabbits and higher growth rate in rabbits born in summer and in autumn than in the other two previous seasons (387 and 393 g vs 323 and 313 g, p<0.001; 14.9 and 15.3 g/d vs 12.3 and 11.8 g/d, p<0.001; respectively).
Individual weight at 21st d of age is the variable most related to milk output because up to this time the pups consume practically only milk, which contributes to both their growth and development and their survival before weaning (Villalobos et al., 2010). Moreover, individual milk intake is highly dependent on the size of the suckling litter and the milk produced by the doe, which latter is reduced by high environmental temperatures and this reduces doe feed intake in turn.
The best growth of the rabbits born in summer and autumn was ascribed to the significantly lower litter size in these two SB, which provided more milk/pup, and also to the more favourable environmental temperatures during growth, particularly for rabbits born in autumn. On the contrary, the spring SB provided the worst litter performance at 21st d of age because both litters and does had to cope with rising environmental temperatures.
The growth performance of young rabbits at weaning and pre-weaning mortality are summarized in Table 4. Litter size, litter weight, and individual body weight, and daily individual growth rate from 22 d to weaning and during the pre-weaning period were affected only by SB. The litter size of rabbits at weaning was still higher in winter than in summer SB (8.4 vs 6.0 rabbits/litter; p<0.001).
Individual weaning weight was higher in rabbits born in summer than in winter and spring (1,142 vs 1,017 and 971 g, respectively; p<0.05) but the litter weight at weaning was higher in rabbits born in winter due to litter sizes that were higher than those born in spring and summer (8,490 vs 7,240 and 6,789 g/litter; p<0.05), in this way confirming the results found in literature (Ayyat and Marai, 1998; Marai et al., 2002; Marai et al., 2006).
The best individual growth rate during 22 d-weaning was observed in rabbits born in winter; the worst was seen in those born in spring (29.0 vs 23.7 g/d; p<0.05) due to higher solid food intake at lower environmental temperatures.
The individual growth rate of rabbits during the entire pre-weaning period was highest in rabbits born in summer and in winter because they missed the hot season, and was lowest in those born in spring (22.8 and 21.2 vs 19.0 g/d; p<0.01) when young rabbit growth was negatively affected by the heat. This worst rabbit pre-weaning performance observed in the spring SB has also been recently reported by Villalobos et al. (2010).
After evaluating the reproductive and productive performance of does and their litters it can be concluded that the two sire breeds tested (Vienna Blue and Burgundy Fawn) are comparable and seem to be suitable to the organic production system. The season of birth affected most of the performances of does and young rabbits reared with the organic farming system, and the rabbits seemed better suited to these rearing conditions during winter than in other seasons. The results obtained seem promising: after an initial difficulty in coping with rising temperatures in spring, both does and litters showed a moderately good ability in adapting to adverse environmental conditions, and this was probably due to the genetic origin.
ACKNOWLEDGMENTS
Research funded by MIUR (PRIN 2002 - Prot. 2002078279_004). The certified organic farm âNoi e la Naturaâ http://www.noielanatura.it is greatly acknowledged.
Table 1.
Chemical composition of the organic diet fed to does and litters (% as fed)
| Organic diet1 | |
|---|---|
| Dry matter (DM, %) | 92.01 |
| Crude protein (CP, %) | 13.2 |
| Ether extract (EE, % | 3.9 |
| Crude fibre (CF, %) | 14.4 |
| Ash (%) | 8.7 |
| NDF (%) | 29.1 |
| ADF (%) | 17.0 |
| ADL (%) | 3.0 |
| Ca (%) | 1.81 |
| P (%) | 0.60 |
| Gross energy (GE, MJ/kg) | 16.35 |
Table 2.
Effect of sire genetic origin (SGO), season of birth (SB) and parity order (P) on the reproductive performances of rabbit does (n = 58) and on litter size and weight at birth
| Sire genetic origin (SGO) | Season of birth (SB) | Parity (P) | p-value | RMSE1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| Vienna Blue | Burgundy Fawn | Winter | Spring | Summer | Autumn | Nulliparous | Primiparous | Multiparous | SGO | SB | P | ||
| Observations (n) | 61 | 60 | 35 | 43 | 29 | 14 | 42 | 25 | 54 | ||||
| Fertility rate (%) | 84.0 | 82.8 | - | - | - | - | - | - | - | ns | - | - | 19.3 |
| Average liveweight at delivery (g) | 4,205 | 4,297 | 4,289 | 4,295 | 4,208 | 4,214 | 4,122b | 4,280ab | 4,352a | ns | ns | * | 423 |
| Interval between deliveries (d) | 62.40 | 67.40 | 67.1 | 72.8 | 61.3 | 58.4 | 64.2 | 61.8 | 68.7 | ns | ns | ns | 17.2 |
| LW variation between deliveries (%) | â0.07 | â2.70 | 2.75a | 0.22a | â0.33ab | â8.18b | 0.41 | â1.52 | â3.05 | ns | * | ns | 5.89 |
| Total born/delivery (n) | 8.40 | 8.30 | 9.5a | 8.6ab | 7.8b | 7.5b | 8.0B | 7.8B | 9.4A | ns | * | ** | 2.32 |
| Born alive/delivery (n) | 8.30 | 8.30 | 9.4a | 8.5ab | 7.8b | 7.5b | 8.0b | 7.8b | 9.1a | ns | * | * | 2.28 |
| Still-born/delivery (n) | 0.10 | 0.06 | 0.14 | 0.17 | 0.00 | 0.01 | 0.05 | 0.00 | 0.24 | ns | ns | ns | 0.73 |
| Milk output at 21st d (g) | 241 | 241 | 251 | 227 | 223 | 264 | 226b | 274a | 224b | ns | ns | * | 69 |
| Litter weight of born alive (g) | 501 | 501 | 535 | 503 | 460 | 505 | 479b | 478b | 546a | ns | ns | * | 124 |
| Individual weight of born alive (g) | 62.5 | 64.1 | 58.1b | 62.2ab | 63.6ab | 69.1a | 61.8 | 67.4 | 60.6 | ns | * | ns | 12.0 |
| Litter size at crossfostering (n) | 8.80 | 8.30 | 9.6A | 8.9AB | 7.9B | 7.9B | 8.2 | 8.4 | 9.1 | ns | ** | ns | 1.8 |
Table 3.
Effect of genetic origin (SGO), of season of birth (SB) and parity order (P) on the litter size and weight at 21 d
| Sire genetic origin (SGO) | Season of birth (SB) | Parity (P) | p-value | RMSE1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| Vienna Blue | Burgundy Fawn | Winter | Spring | Summer | Autumn | Nulliparous | Primiparous | Multiparous | SGO | SB | P | ||
| Litter (n) | 55 | 50 | 31 | 40 | 21 | 13 | 38 | 22 | 45 | ||||
| Litter size at 21 d (n) | 8.1 | 7.6 | 8.8A | 8.2AB | 6.9C | 7.5BC | 7.7 | 7.8 | 8.0 | ns | ** | ns | 1.68 |
| Litter weight at 21 d (g ) | 2,697 | 2,619 | 2,778a | 2,487b | 2,545ab | 2,823a | 2,577 | 2,738 | 2,660 | ns | * | ns | 454 |
| Individual weight at 21 d (g) | 342 | 365 | 323B | 313B | 387A | 393A | 351 | 367 | 342 | ns | *** | ns | 65 |
| Daily individual growth rate 0 to 21 d (g/d) | 13.2 | 14.0 | 12.3B | 11.8B | 14.9A | 15.3A | 13.6 | 14.3 | 12.9 | ns | *** | ns | 2.97 |
Table 4.
Effect of sire genetic origin (SGO), of season of birth (SB) and parity order (P) on the litter size, weight and mortality at weaning
| Sire genetic origin (SGO) | Season of birth (SB) | Parity (P) | p-value | RMSE1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| Vienna Blue | Burgundy Fawn | Winter | Spring | Summer | Autumn | Nulliparous | Primiparous | Multiparous | SGO | SB | P | ||
| Litter (n) | 55 | 50 | 31 | 40 | 21 | 13 | 38 | 22 | 45 | ||||
| Weaning age (d) | 46.9 | 45.9 | 44.9 | 47.5 | 47.0 | 46.2 | 45.8 | 46.5 | 46.9 | ns | ns | ns | 6.2 |
| Litter size at weaning (n) | 7.4 | 7.0 | 8.4A | 7.5AB | 6.0C | 7.0BC | 7.2 | 6.9 | 7.6 | ns | *** | ns | 1.76 |
| Litter weight at weaning (g) | 7,772 | 7,184 | 8,490a | 7,240b | 6,789b | 7,393ab | 7,221 | 7,377 | 7,837 | ns | * | ns | 2,086 |
| Individual weight at weaning (g) | 1,054 | 1,040 | 1,017b | 971b | 1,142a | 1,059ab | 1,011 | 1,087 | 1043 | ns | * | ns | 181 |
| Daily individual growth rate 22 d-weaning (g/d) | 26.5 | 25.5 | 29.0a | 23.7b | 27.0ab | 24.1ab | 25.4 | 25.7 | 26.8 | ns | * | ns | 7.48 |
| Daily individual pre-weaning growth rate (g/d) | 21.0 | 21.0 | 21.2A | 19.0B | 22.8A | 21.1AB | 20.6 | 21.8 | 20.7 | ns | ** | ns | 3.49 |
| Pre-weaning mortality (%) | 15.8 | 16.3 | 14.8 | 15.0 | 20.0 | 14.3 | 11.6 | 20.6 | 15.9 | ns | ns | ns | 15.8 |
REFERENCES
AIAB. 2012. Associazione Italiana per l'Agricoltura Biologica - Disciplinare garanzia AIAB Italia. (http://www.aiab.it)
AOAC. 2000. Official methods of analysis. 17th ednAssociation of Official Analytical Chemists; Gaithersburg, Maryland:
Ayyat MS, Marai IFM, El-Sayiad GHA. 1995. Genetic and nongenetic factors affecting milk production and preweaning litter traits of New Zealand White does, under Egyptian conditions. World Rabbit Sci 3:119â124.
Ayyat MS, Marai IFM. 1998. Evaluation of application of the intensive rabbit production system under the subtropical conditions of Egypt. World Rabbit Sci 6:213â217.
Castellini C, Dal Bosco A, Arias-Alvarez M, Lorenzo PL, Cardinali R, Rebollar PG. 2010. The main factors affecting the reproductive performance of rabbit does: a review. Anim Reprod Sci 122:174â182.


Cavani C, Bianchi M, Lazzaroni C, Luzi F, Minelli G, Petracci M. 2000. Influence of type of rearing, slaughter age and sex on fattening rabbit: II Meat quality. World Rabbit Sci 8:567â572.
Cavani C, Bianchi M, Petracci M, Toschi TG, Parpinello GP, Kuzminsky G, Morera P, Finzi A. 2004. Influence of open-air rearing on fatty acid composition and sensory properties of rabbit meat. World Rabbit Sci 12:247â258.

Council Regulation (EC) No. 1804/1999 supplementing Regulation (EEC) No. 2092/91 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs to include livestock production. CELEX-EUR Official Journal L 222 24August. 1999. 1â28. Website: europa.eu.eur18524.doc.
DâAgata M, Preziuso G, Russo C, Dalle Zotte A, Mourvaki E, Paci G. 2009. Effect of an outdoor rearing system on the welfare, growth performance, carcass and meat quality of a slow-growing rabbit population. Meat Sci 83:691â696.


Dal Bosco A, Castellini C, Bernardini M. 2000. Productive performance and carcass and meat characteristics of cage- or pen-raised rabbits. World Rabbit Sci 8:579â583.
Dal Bosco A, Castellini C, Mugnai C. 2002. Rearing rabbits on wire net floor or straw litter: behaviour, growth and meat qualitative traits. Livest Prod Sci 75:149â156.

Dal Bosco A, Castellini C, Mugnai C. 2003. Influenza del sistema di allevamento sul metabolismo energetico dei muscoli e sulla stabilitĂ ossidativa della carne di coniglio. Riv Coniglicoltura 4:46â47.
Dalle Zotte A, Princz Z, Metzger Sz, SzabĂł A, Radnai I, BirĂł-NĂŠmeth E, Orova Z, SzendrĹ Zs. 2009. Response of fattening rabbits reared under different housing conditions. 2. Carcass and meat quality. Livest Sci 122:39â47.

Fortun-Lamothe L. 2006. Energy balance and reproductive performance in rabbit does. Anim Reprod Sci 93:1â15.


Hansen PJ. 2007. Explotation of genetic and physiological determinants of embryonic resistance to elevated temperature to improve embryonic survival in dairy cattle during heat stress. Theriogenology 68:Suppl 1S242âS249.


ISO. 1998. Animal feeding stuffs, animal products and faeces or urine. Determination of gross calorific value - Bomb calorimetric method. Reference number 9831
Khalil MH, Al-Saef AM. 2008. Methods, criteria, techniques and genetic responses for rabbit selection: a review. In : Proceedings of 9th World Rabbit Congress; Verona, Italy. p. 1â18.
Lacetera N, Bernabucci U, Ronchi B, Nardone A. 2003. Physiological and productive consequences of heat stress. The case of dairy ruminants. Proceedings of the Symposium on interaction between Climate and Animal Production: EAAP Technical Series 7:45â60.

Lebas F, Coudert P, Rouvier R, De Rochambeau H. 1986. The rabbit. Husbandry, Health and Production. FAO. Ed.; Rome, Italy:
Marai IFM, Habeeb AAM, Gad AE. 2002. Rabbitâs productive, reproductive and physiological performance traits as affected by heat stress: a review. Livest Prod Sci 78:71â90.

Marai IFM, Rashwan AA. 2004. Rabbits behavioural response to climatic and managerial conditions - a review. Arch. Tierz Dummerstorf 47:469â482.

Marai IFM, Askar AA, Bahgat LB. 2006. Tolerance of New Zealand White and Californian doe rabbits at first parity to the sub-tropical environment of Egypt. Livest Sci 104:165â172.

Nardone A, Ronchi B, Lacetera N, Ranieri MS, Bernabucci U. 2010. Effects of climate changes on animal production and sustainability of livestock systems. Livest Sci 130:57â69.

Ozimba CE, Lukefahr SD. 1991. Comparison of rabbit breed types for postweaning litter growth, feed efficiency and survival performance traits. J Anim Sci 69:3494â3500.


Paci G, Lisi E, Maritan A, Bagliacca M. 2003. Reproductive performance in a local rabbit population reared under organic and conventional system. Ann. Fac. Med. Vet Pisa 56:115â125.
Paci G, Lisi E, Cini A, Bagliacca M. 2004. Tecniche di allevamento e caratteristiche di conigli biologici prodotti in unâazienda certificata della Toscana. Riv Coniglicoltura 5:14â17.
Paci G, Schiavone A, Lisi E, Peiretti PG, Bagliacca M, Mussa PP. 2005. Meat quality characteristics in local population of rabbit reared with organic system. In : Proceedings of the 16th ASPA Congress; 4:p. 562
Pascual JJ, Cervera C, Fenandez-Carmona J. 2000. The effect of dietary fat on he performance and body composition of rabbits in their second lactation. Anim Feed Sci Technol 86:191â203.

Piles M, Tusell Ll, GarcĂa-TomĂĄs M, Baselga M, GarcĂa-Ispierto I, Rafel O, Ramon J, LĂłpez-Bejar M. 2008a. Genotype X Sperm dosage interaction on reproductive performance after artificial insemination. 1. Male fertility. In : Proceedings of 9th World Rabbit Congress; Verona, Italy. p. 1â18.
Piles M, Tusell Ll, GarcĂa-TomĂĄs M, Baselga M, GarcĂa-Ispierto I, Rafel O, Ramon J, LĂłpez-Bejar M. 2008b. Genotype X Sperm dosage interaction on reproductive performance after artificial insemination. 2 Male litter size. In : Proceedings of 9th World Rabbit Congress; Verona, Italy. p. 227â232.
Pla M. 2008. A comparison of the carcass traits and meat quality of conventionally and organically produced rabbits. Livest Prod Sci 115:1â12.

Princz Z, Dalle Zotte A, Metzger Sz, Radnai I, BirĂł-NĂŠmeth E, Orova Z, SzendrĹ Zs. 2009. Response of fattening rabbits reared under different housing conditions. 1. Live performance and health status. Livest Sci 121:86â91.

Rebollar PG, Perez-Cabal MA, Pereda N, Lorenzo PL, Arias-Alvarez M, Garcia-Rebollar P. 2009. Effects of parity order and reproductive management on the efficiency of rabbit productive systems. Livest Sci 121:227â233.

Rommers JM, Kemp B, Meijerhof R, Noordhuizen JPTM. 1999. Rearing management of rabbit does: a review. World Rabbit Sci 7:125â138.

SAS Institute Inc. 2007. JMP7 userâs guide: Version 7. 2nd ednSAS Institute Inc; Cary, North Carolina:
SzendrĹ Zs, Matics Z, GerencsĂŠr Z, Nagy I, Lengyel M, Horn P, Dalle Zotte A. 2010. Effect of dam and sire genotypes on productive and carcass traits of rabbits. J Anim Sci 88:533â543.


SzendrĹ Zs, SzendrĹ K, Dalle Zotte A. 2012. Management of reproduction on small, medium and large rabbit farms: a review. Asian-Aust J Anim Sci 25:738â748.



Theau-Clement M. 2007. Preparation of the rabbit doe to insemination: a review. World Rabbit Sci 15:61â80.

Villalobos O, Guillen O, Garcia J. 2010. Effect of breeding system, cycle and cage size during fattening on rabbit doe and growing rabbit performance under heat stress. Animal 4:1568â1576.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





