 |
 |
Abstract
This study was to evaluate the feasibility of recycling the solids separated from swine wastewater treatment process as a fuel source for heat production and to provide a data set on the gas emissions and combustion properties. Also, in this study, the heavy metals in ash content were analyzed for its possible use as a fertilizer. Proximate analysis of the solid recovered from the swine wastewater after flocculation with organic polymer showed high calorific (5,330.50 kcal/kg) and low moisture (15.38%) content, indicating that the solid separated from swine wastewater can be used as an alternative fuel source. CO and NOx emissions were found to increase with increasing temperature. Combustion efficiency of the solids was found to be stable (95 to 98%) with varied temperatures. Thermogravimetry (TG) and differential thermal analysis (DTA) showed five thermal effects (four exothermic and one endothermic), and these effects were distinguished in three stages, water evaporation, heterogeneous combustion of hydrocarbons and decomposition reaction. Based on the calorific value and combustion stability results, solid separated from swine manure can be used as an alternative source of fuel, however further research is still warranted regarding regulation of CO and NOx emissions. Furthermore, the heavy metal content in ash was below the legal limits required for its usage as fertilizer.
The growing demand for meat and meat production has been on a steady increase in both developed and developing countries (Yetilmezsoy et al., 2009). Therefore, the concentration of animal production systems and consequently animal wastewater production has proportionately increased. In many cases, the production of manure from one or more animal species is in excess of what can be safely applied to farmland in accordance with nutrient management plans, and stock piled waste poses economic and environmental liabilities. This biowaste contributes to eutrophication of water bodies, spread of pathogens, production of phytotoxic substances and air pollution with the release of CH4 (a green house gas), NH3, H2S, amides, volatile organic acids, mercaptans, esters, and other compounds (Sweeten et al., 2003; Zhou et al., 2012). Therefore, effective treatment of animal manure must be implemented before its discharge into the environment.
Approaches currently in use for proper manure management are biological nutrient removal, anaerobic digestion (generate energy), and composting (solid fertilizer) (Gonzalez-Fernandez et al., 2008). The solid-liquid separation is critical for the accomplishment of the above mentioned techniques. In order to improve the settling characteristics of solids in manure, chemical methods are commonly being used and many inorganic chemicals (ferric chloride and aluminium sulphate) (Zhang and Lei, 1998) and organic polymers (polyacrylamides and chitosan) have been used as coagulants and flocculants for separation augmentation. Among the organic polymers, polyacrylamides have been widely studied for swine wastes (Vanotti and Hunt, 1999; Ebeling et al., 2005). These polymers are proven to be effective for flocculating suspended solids (SS) and separating organic nutrients from swine wastewater with low dosage demand. After separation of solids, the wastewater is purified by physical and biological processes before being discharged and the leftover solid is passed through a composting process. Their utilization as a solid fuel source would be a good method of management/or treatment of the separated solids especiaaly in view of rising energy prices. Furthermore, if the amount of heavy metals in the resulting ash after combustion were less than the prevailing regulations, it could be used as a fertilizer.
The development of thermal treatment of biomass waste was driven by restrictions of gas emission standards and requirements of residue qualities by legislative regulations due to concerns over public health and the environment (Freeman, 1997; Frey et al., 2003). Recent research efforts have shown that biomass (manure) fuels are considered to be environmentally friendly for several reasons. Biomass wastes bring additional greenhouse gas mitigation by avoiding CH4 release from land filling (Hein and Bemtgen, 1998; Spliethoff and Hein, 1998). There is no net increase in CO2 as a result of burning a biomass fuel because biomass consumes the same amount of CO2 from the atmosphere during growth as it is released during combustion (Easterly and Brunham, 1996).
In this study to determine the feasibility of recycling of the solids separated from the swine wastewater treatment process their energy content, efficiency of complete combustion and gas emissions properties during combustion were studied. Also the heavy metal content before and after combustion was analyzed to determine whether the generated ash can be used as fertilizer.
Slurry form swine (feeder to finish) wastewater was collected from the university farm and kept at 4°C until used. For separation of the solids from wastewater, the slurry was flocculated with 100 times diluted polymer (commercial organic polymer, Nalco 855) at a rate of 50 mg/L, and then the mixture was stirred for 30 min and kept for 1 h for settlement. The separated solids from the swine wastewater were dried in an open plastic vessel for 3 d under natural conditions (i.e., in an experimental shed) for the evaluation of the solid waste to be recycled as a solid fuel.
The wastewater collected after solid separation was analyzed for total solid (TS), suspended solids (SS), ammonia-nitrogen (NH4-N), orthophosphate (OP), total Kjeldahl nitrogen (TKN), total phosphate (TP), and total soluble organic carbons (TOCs). NH4-N, OP, TKN, and TP were analyzed with an auto water analyzer (Quick Chem 8500, LACHAT), and TOCs wereanalyzed using a Total Organic Carbon Analyzer (Shimadzu, TOC-5000A). The separated solid after natural drying was measured for moisture, TS, volatile solid (VS), ash and energy level. For estimation of moisture content, the solids were dried in electric oven at approximately 105°C for 24 h. For VS and ash, the weight of solids was measured before and after ignition at 550°C for 4 h and the VS being the difference between the dried solids and the ash. All the analyses were carried out according to APHA (2005) in triplicate. The calorific value of the solid waste was measured by burning the weighed (0.5 g) sample in an oxygen bomb calorimeter according to ASTM E-711.
A combustion experiment was done with a small boiler furnace consisting of fine grid body, fuel supply, combusting chamber and the ash pan. The furnace had an area of 66 to 132 m2. Boiler dimensions (W×L×H) were 650×1,230×1,600 (mm) with a capacity of 110 L for liquid and 210 kg for solid. Operating conditions during combustion are given in Table 1. The analysis of the flue gas composition was carried out immediately using “Combustion Gas Analyzer” (ECOM Ltd America, ECOM – AC 2.), and a multi-component gas analyzer was used for online measurements of CO, NO, SO2 and O2. Table 2 shows the ranges for the different gas components. The microprocessor calculated CO2 and combustion efficiency.
The separated solids from swine wastewater were subjected to TG and DT analysis in nitrogen and air atmosphere using STA 409 PC Luxx® Simultaneous TGA-DTA analyzer, where the mass loss, TG, and temperature changes were recorded simultaneously. Thermogravimetric curves were obtained at a heating rate of 10°C/min. Purified nitrogen and air at a flow rate of around 50 ml/min was used as the purge gas to provide an inert/oxidative atmosphere for pyrolysis and combustion, respectively. Mineral analysis of the solid and ash were examined through the optical emission spectroscopy.
Content of solids in wastewater is an important parameter for the control of flocculation treatments (Vanotti et al., 2002). In order to remove solids from swine wastewater, which are responsible for the oxygen requirement in further treatment processes, the wastewater was flocculated with polymer. As shown in Table 3, the removal efficiencies of TS and SS were 28.19 and 73.57%, respectively. Vanotii and Hunt (1999) obtained removal of 96% for SS, using 200 ppm of polyacrylamide (PAM) on a raw wastewater of 6.7 g/L TS and Gonzalez-Fernandez (2008) reported 79% of SS removal with 120 ppm of PAM dosage. In this study, a lower removal efficiency was achieved probably due to the lower concentration of polymer used (<50 ppm). In general, removal efficiency of TS and SS depends on their initial concentration in raw wastewater as well as on type and concentration of polymer used.
Ammonia-nitrogen was found to increase in the effluent, probably due to the chemical composition of the polymer, whereas OP, TKN, TP and TOCs were decreased in the effluent, showing 45.53, 12.10, 74.23 and 59.41% removal, respectively. With respect to phosphorus, Gonzalez-Fernandez (2008) and Timby et al. (2004) concluded that flocculation with PAM did not affect soluble phosphorus in dairy and swine wastewater. None of the authors working on swine wastewater have reported soluble phosphorus removal by flocculation treatment, except for Vanotti et al. (2003) who found a 17% removal of soluble P with flocculation and a separation treatment. This removal was unexpected since flocculation is supposed to affect the non soluble phosphorus, but some researchers working with a different kind of wastewater have observed this phenomenon when using polyacrylamides as flocculation aids (Ebeling et al., 2005) and the removal of soluble P was attributed to the removal of TS and SS. This fact may explain the results of soluble P removal in our study. A similar trend was also observed in case of TOCs.
The main physio-chemical parameters which determine the potential recovery of energy from solid wastes are density, moisture content and calorific value (Houshfar et al., 2010). Wastes of high density reflect a high proportion of biodegradable organic matter and moisture. High moisture (>45%) makes the waste rather unsuitable for thermo-chemical conversion for energy recovery as heat must first be supplied to remove moisture. The results obtained in the present study are shown in Table 4. The calorific value of the solid is much higher (5,330.40 kcal/kg) compared to those of horse manure mixed with wood shavings (4,626.44 kcal/kg) having moisture content of 57% (Lundgren and Pettersson, 2009), poultry litter having moisture content of 15.02% and calorific content of 1,440.24 kcal/kg (Zhu et al., 2005) and feedlot manure having 4,991.87 kcal/kg calorific value (Sweeten et al., 2003). Therefore, it appears that the sludge or solids produced from the swine wastewater treatment process y using an organic polymer could be a useful source for energy generation. The TS, organic and inorganic content were found to be 84.61, 67.10 and 32.89%, respectively.
Both CO and NOx emissions depend upon matter characteristics, operating condition temperature and excess air etc. CO and NOx fluctuate more with higher sludge solid content (TS) (Dangtran et al., 2000). Figure 1b shows that with an increase in temperature from 100 to 240°C, there was an increase in CO (600 to 880 ppm) and NOx (375 to 420 ppm) emissions. In the case of CO, an increase in emission was observed in steady intervals at 140 to 160 and 180 to 200°C, whereas in the case of NOx increased emission was observed till 180°C and thereafter emission was stable. Gases emitted from the combustion chamber are mainly NO2 and NO, which are collectively defined as NOx. NOx emission can be categorized as thermal NOx, prompt NOx and fuel NOx. During gas fuel or denitrified fuel combustion, thermal NOx and prompt NOx are produced. If nitrogen content is >3% as in case of heavy oil or charcoal, then fuel NOx is main cause of NOx production. In this experiment, NO produced from the solids was less than 400 ppm which is comparatively lower than the standard environmental value (500 ppm).
The slight increase in NOx with increase in temperature could be due to the release of fuel-N from NH3 groups and thus enhanced formation of NOx coming from a small percentage of nitrogen bound in the sludge (Zhu et al., 2005). Similar results were also obtained by Zhu and Lee (2005) while assessing temperature effect on NOx emission of sawdust, poultry litter and manure co-combusted with natural gas.
From Figure 1a, it can be noticed that air excess ratio was increasing with the increase in temperature. With increase in the combustion temperature, CO concentration value was observed to be high (Figure 1b). CO concentration in flue gas heavily relies on air fuel ratio (equivalence ratio; Ø). With high O2 content there is complete oxidation of CO to CO2, but with a lack of O2 a large amount of CO is produced. In other words, if Ø >1, there is significant increase in CO emission conversely if Ø <1 only small concentration of CO is formed. Hence, for low CO emission a high air-fuel ratio is required.
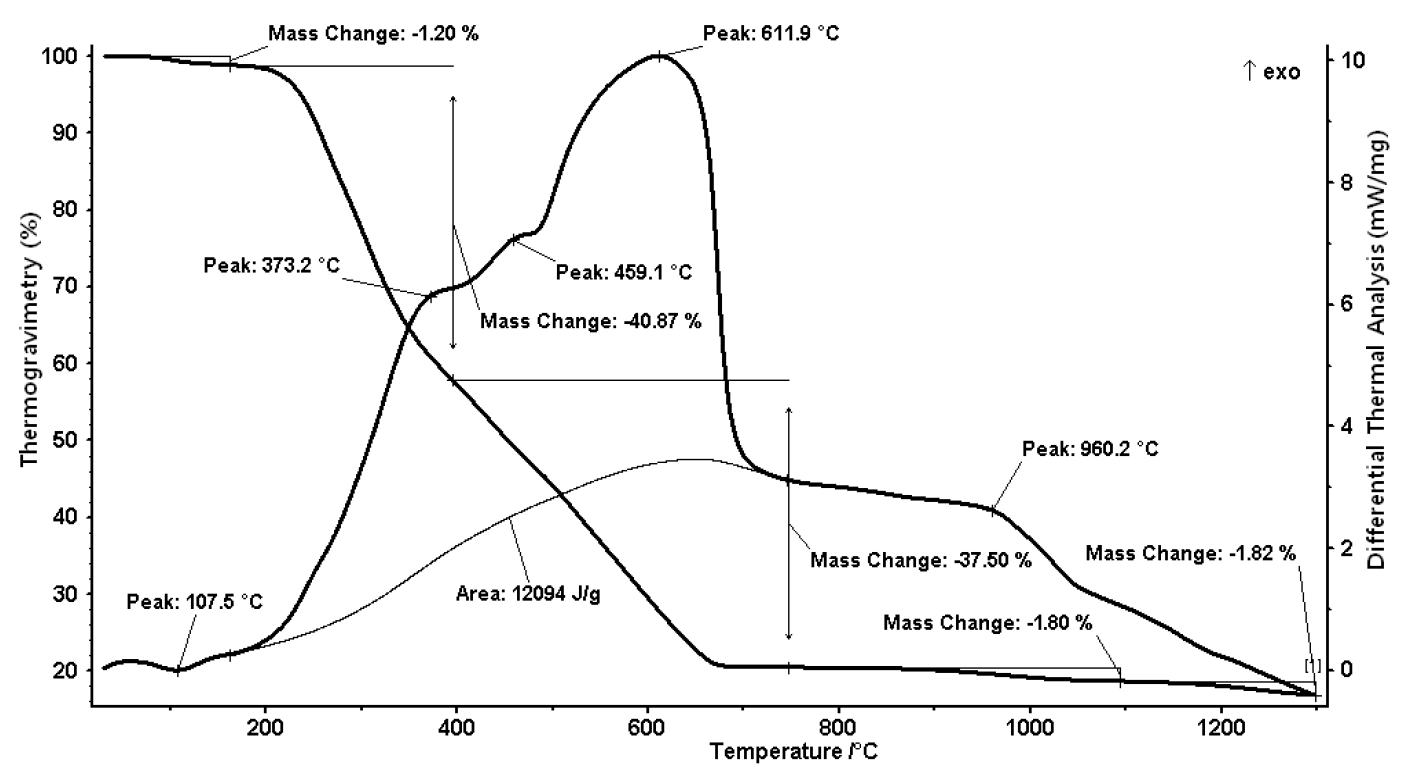
TG analysis of the separated solid from swine wastewater at 10°C/min is presented in Figure 2.Concerning the TG data five thermal effects were clearly identified. The correlation between TG data and DTA curves contributed to the interpretation of these thermal effects. Four exothermal (in the temperature ranges 373.2, 459.1, 611.9 and 960.2°C) and one endothermal (107.5°C) peaks were determined during TG-DTA anlaysis. The peak at 107.5°C was caused by the volatilization of water in the solids (Iordanidis et al., 2001; Xiu et al., 2012). DTA curves indicate that it was an endothermic stage under inert conditions. Mass change of 1.20% was observed at this stage. Following this a huge mass change of 40.87% and 37.50% was observed between 200 to 400°C and 400 to 600°C, respectively. Consequently, 80% mass change was noticed in the temperature range of 200 to 680°C. The DTA curve displayed two exothermic peaks in this region, which is probably due to the decomposition reaction of the heavy components (hydrocarbons) in the solids (Xiu et al., 2012). After this intensive mass change, low mass changes of 1.80 and 1.87 were observed in the temperature range of 900 to 1,080 and 1,180 to 1,290°C, respectively. A total of 83.19% mass change was observed overall. On the basis of data obtained, the combustion of the separated solid from swine wastewater can be divided into three phases: In the first phase, water and light compounds volatilized and were oxidized, which occurred before 200°C; in the second phase, the heterogeneous combustion occurred between the heavy hydrocarbons and oxygen; this lead to the main weight loss in the second region. DTA curve analysis around 611.9°C showed an exothermic peak having a calorific value of 2,889.29 cal/kg. In the third stage, the char that was formed during the previous process of evaporating and cracking was observed by two peaks in the temperature of more than 900°C.
Optical emission spectrometric analysis was done to analyze inorganic components of the solid separated from swine wastewater and of bottom ash after combustion (Table 5). The analysis of the major inorganic components of the bottom ash compared with the solid showed decreased concentration of SO3, Cl and K2O in bottom ash, whereas substantial increase in MgO, SiO2, and P2O5 was noticed after combustion.
Coal ash showed lower amount of Na2O, K2O and higher content of Al2O3, SiO2 and Fe2O3 compared to horse manure and the bottom ash. Ca content of the bottom ash was higher than that of the horse fuel mixture and comparable to coal ash, thus can have higher capacity of self hardening due to formation of limestone (in humid conditions) on reaction with carbon dioxide. Nitrogen content is completely absent from the ash. The phosphorus content in the ash is high, and thus can replenish the lost phosphorus content of the soil. Since, heavy metals are an important issue with regard to assessing the quality of ash as possible fertilizer, the heavy metal content in the ash as well solid fuel was analyzed (Table 6). As and Se decreased in the bottom ash while Cd, Cr, Cu, Co, Mo, Ni, Pb and Zn increased. On comparing the legal limits stated by DuMV (2003), the ash contained much lower heavy metal content than the limit values. Sweeten et al. (2003) and Lundergen and Petterson (2009), reported that heavy metal contents in ash must be lower than the legal limits for its use as fertilizer or before being recycled to forests.
From the experimental studies on swine wastewater carried out in the present work the following conclusions can be drawn: i) The energy content of the solid or sludge separated from swine wastewater using organic polymer was 5,330.40 kcal/kg. The CO and NOx emission was found to increase with increasing temperature. The O2 content and excess air ratio was higher and was responsible for high CO and NOx emission, ii) The TG-DTA analysis suggested that the combustion of the sludge separated from swine wastewater treatment plants could be processed in three stages. First stage and third stage showed lower mass loss due to water evaporation and decomposition, whereas in the second stage a high mass loss of 80% was observed, iii) Ash contained high fertilizer sources such as MgO, P2O5, CaO and K2O, while heavy metal content was very low compared to legal limits and iv) The sludge produced by using organic polymer in swine wastewater treatment process can be used as fuel with high combustion efficiency, however, CO and NOx emission should be regulated.
ACKNOWLEDGEMENT
This research was performed with the support of Rural Development Administration (RDA), Korea.
Figure 1
Fuel characteristics of solid wastes as function of temperature. (a) Air excess ratio (%); (b) CO and NOx concentration; (c) CO2 and O2 concentration; (d) Combustion efficiency.
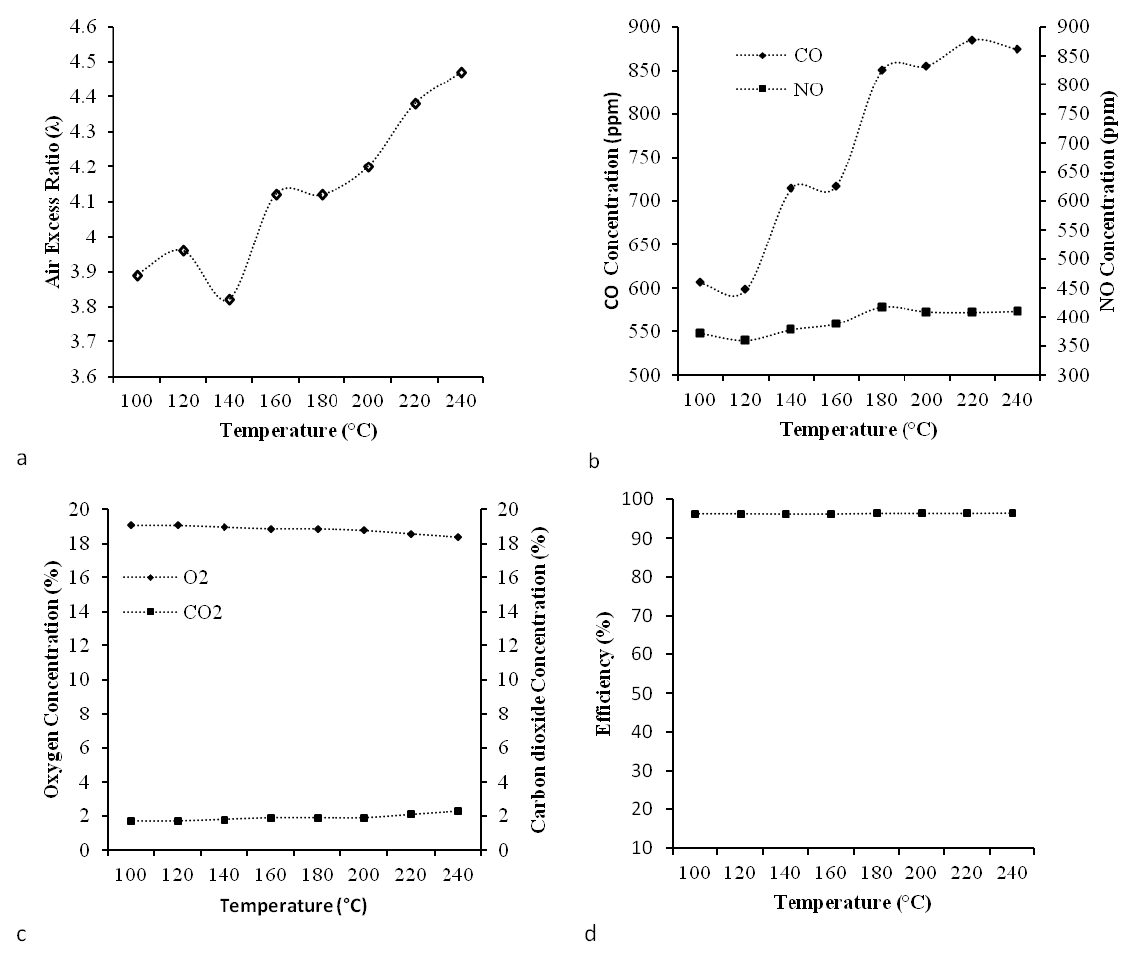
Table 1
Operational condition during combustion
| Parameters | Conditions |
|---|---|
| Average combustion temperature | 100–300°C |
| Fuel moisture content | 15.38% |
| Air ratio | 3.89–4.47 |
Table 2
Measuring ranges for different gas components
Table 3
Characteristics of the swine wastewater before and after solid separation
Table 4
Proximate analysis of the solid waste
| Proximate analysis | Solid (this study) | Horse manurea | Poultry littera | Feedlot manurea |
|---|---|---|---|---|
| Moisture content (%) | 15.38±0.12 | 57.00 | 15.02 | 38.60 |
| Calorific value (kcal/kg) | 5,330.40±34.20 | 4,626.44 | 1,440.24* | 4,991.87* |
a Data from Lundgren and Pettersson, 2009 (converted to kcal/kg).
Table 5
Comparison of major inorganic components in solid waste before and after burning
| Components |
Mass (%)
|
|||
|---|---|---|---|---|
| Solid waste | Bottom ash | Horse manure* | Coal ash** | |
| Na2O | 2.10 | 2.08 | 2.59 | 0.94 |
| MgO | 5.39 | 7.42 | 8.85 | 5.53 |
| Al2O3 | 0.95 | 1.38 | 7.75 | 19.08 |
| SiO2 | 4.31 | 6.24 | 42.6 | 28.7 |
| P2O5 | 15.80 | 24.0 | 4.27 | – |
| SO3 | 13.0 | 8.95 | – | 9.86 |
| Cl | 6.73 | 3.29 | – | – |
| K2O | 19.1 | 12.5 | 11.5 | 0.5 |
| CaO | 25.7 | 25.7 | 15.4 | 27.9 |
| TiO2 | 0.13 | 0.14 | 0.44 | 1.34 |
| Fe2O3 | 3.40 | 3.92 | 4.24 | 5.58 |
Table 6
Heavy metal contents in the bottom ash and in the solid waste (mg/kg) and its comparison with legal limit stated by Dungemittelverordnung
| Element (mg/kg) | Bottom ash | Solid waste | Limit values according to DuMV, 2003 |
|---|---|---|---|
| As | 0.011 | 0.017 | – |
| Cd | ND* | ND | 1.5 |
| Cr | 0.111 | 0.015 | 2.0 |
| Cu | 9.868 | 2.036 | 70 |
| Co | 0.048 | 0.003 | – |
| Hg | ND | ND | – |
| Mo | 0.125 | 0.016 | – |
| Ni | 0.196 | 0.032 | 80 |
| Pb | 0.067 | 0.026 | 150 |
| Se | ND | ND | – |
| Zn | 31.279 | 5.617 | 1,000 |
REFERENCES
APHA. 2005. Standard methods for the examination of water and wastewater. 21st ednAmerican Public Health Association; Washington, DC, USA:
Dangtran K, Mullen JF, Mayrose DT. 2000. A comparison of fluid bed and multiple hearth sludge incineration. In : Presented at the 14th Annual Residual and Sludge Incinerator Emissions, WERF Project 91-ISP-1;
Decolorization and COD reduction of UASB pretreated poultry manure wastewater by electrocoagulation process: A post-treatment study. J Hazard Mater 162:120–132.


German Fertilizer Regulations Dungemittelverordnung. 2003. Verordnung uber das Inverkehrbringen von Dungemitteln, Bodenhilffftoffen. Kultursubstraten und Pflanzenhilfsmitteln.
Easterly JL, Brunham M. 1996. Overview of biomass and waste fuel resources for power production. Biomass Bioenergy 10:79–92.

Ebeling JM, Rishel KL, Sibrell PL. 2005. Screening and evaluation of polymers as flocculation aids for the treatment of aqualculture effluents. Aquac Eng 33:235–249.

Ferrer M, Orus F, Monge E. 2000. Determinacion de formas nitrogenadas en estiercol fluido de porcino (EFP) pro distinos metodos analiticos. Anaporc 205:86–101.
Freeman HM. 1997. Standard Handbook of Hazardous Waste Treatment and Disposal. McGraw Hill; NY: 0070220442
Frey HH, Peters B, Hunsinger H. 2003. Characterization of municipal solid waste combustion in a grate furnace. Waste Manag 23:689–701.


Gonzalez-Fernandez C, Nieto-Diez PP, Leon-Cofreces C, Garcia-Encina PA. 2008. Solids and nutrients removal from liquid fraction of swine manure slurry through screening and flocculation treatment and influence of these processes on anaerobic biodegradability. Bioresour Technol 99:6233–6239.


Hein KPG, Bemtgen JM. 1998. EU clean coal technology, co combustion of coal and biomass. Fuel Process Technol 54:159–169.

Houshfar E, Lovas T, Skreiberg OQ. 2010. Detailed chemical kinetics modeling of NOx reduction in combined staged fuel and staged air combustion of biomass. In : 18th European Biomass Conference and Exhibition; Lyon, France. p. 1128–1132.
Iordanidis A, Georgakopoulos A, Markova K, Filippidis A, Kassoli-Fournarraki A. 2001. Application of TG-DTA to the study of Amynteon lignites, northern. Greece Thermo Acta 371:137–141.

Lundgren J, Pettersson E. 2009. Combustion of horse manure for heat production. Bioresour Technol 100:3121–3126.


Spliethoff H, Hein KPG. 1998. Effect of co-combustion of biomass on emissions in pulverized fuel furnaces. Fuel Process Technol 54:189–205.

Sweeten JM, Annamalai K, Thien B, McDonald LA. 2003. Co-firing of coal and cattle feedlot biomass (FB) fuels. Part I. Feedlot biomass (cattle manure) fuel quality and characteristics. Fuel 82:1167–1182.

Timby GG, Daniel TC, McNew RW, Moore PA. 2004. Polymer type and aluminium chloride effect screened solids and phosphorus removal from liquid dairy manure. Appl Eng Agric 20:57–64.

Vanotti MB, Hunt PG. 1999. Solids and nutrient removal from flushed swine manure using polyacrylamides. Trans Am Soc Agric Eng 42:1833–1840.

Vanotti MB, Hunt PG, Szogi A, Humenik F, Millner P, Ellison A. 2003. Solids separation, nitrification-denitrification, soluble phosphorus removal, solids processing system. Proceedings of the North Carolina Animal Waste Management Workshop 30–35.
Vanotti MB, Rashash DMC, Hunt PG. 2002. Solid-liquid separation of flushed swine manure with PAM: effect of wastewater strength. Trans Am Soc Agric Eng 45:1959–1969.

Xiu S, Rojanala HK, Shahbazi A, Finni EH, Wang L. 2012. Pyrolysis and combustion characteristics of Bio-oil from swine manure. J Therm Anal Calorim 107:823–829.

Yetilmezsoy K, Ilhan F, Sapci-Zengin Z, Sakar S, Gonullu MT. 2009. Decolorization and COD reduction of UASB pretreated poultry manure wastewater by electrocoagulation process: A post-treatment study. J Hazard Mat 162:120–132.

Zhang RH, Lei F. 1998. Chemical treatment of animal manure for solid-liquid separation. Trans Am Soc Agric Eng 41:1103–1108.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





