 |
 |
Abstract
The objective of this study was to investigate the effect of rapid chilling (RC) on beef quality and the degradation of cytoskeletal proteins. Twenty Chinese Yellow crossbred bulls were selected and randomly divided into two groups. RC and conventional chilling (CC) were applied to left and right sides of the carcasses respectively after slaughtering. To determine whether electrical stimulation (ES) treatment can alleviate the potential hazard of RC on meat quality, ES was applied to one group. The effects of RC and ES were determined by meat color, shear force and cytoskeletal protein degradation postmortem (PM). The results showed that RC decreased beef tenderness at 1 d and 3 d postmortem, but had no detrimental effect on meat color. Western blotting showed that RC decreased the degradation rate of desmin and troponin-T, but the effects weakened gradually as postmortem aging extended. Degradation rates of both desmin and troponin-T were accelerated by ES. The combination of RC and ES could improve beef color, accelerate degradation rate of cytoskeletal protein and improve beef tenderness.
Chilling processes of livestock carcasses is one of the most important factors that affect product quality and is employed to ensure food safety, extend shelf-life, and reduce shrinkage (Savell et al., 2005). Conventional carcass chilling is a lengthy, energy-expensive process (Janz et al., 2001) and introduces extra microbial hazards and increases evaporative weight loss (Joseph, 1996). RC can increase production efficiency by decreasing the required chilling time and lowering evaporative loss (Janz et al., 2001). However, RC may decrease tenderness and increase the variation in tenderness, both of which influence consumer’s satisfaction for beef palatability (Miller et al., 2001; Savell et al., 2005; White et al., 2006a). Many studies also reported that RC has detrimental effect on beef color, resulting in darker meat color (Janz et al., 2001; Aalhus et al., 2002) and reducing purchase desire of consumers.
Although China has become the third-largest beef producer in the world, some techniques such as electrical stimulation are not used broadly and the tenderness of beef product need to be increased urgently, so the processing technology of beef in China appeals for further improvement. The effects of RC and ES on beef quality of Chinese Yellow crossbred bulls have been reported (Li et al., 2006), however, the effects on meat color have not been reported and mechanisms of tenderizing were not investigated extensively.
It is generally accepted that the degradation of the skeleton proteins plays an important role in the tenderization process (Rhee et al., 2000). Ho et al. (1996) reported that desmin and troponin-T are two of the major cytoskeletal proteins that degrade during postmortem aging. Desmin is one of the transversely running elements that links adjacent myofibrils together, and its degradation causes the loss of transversal alignment of the sarcomeres (Rhee et al., 2000). Troponin-T is part of the regulatory complex that mediates the actin-myosin interaction. The degradation of troponin-T will alter the interactions of thick and thin filaments. The degradation of these two proteins can cause fragmentation of the myofibril and loss of muscle cell integrity, and ultimately contribute to meat tenderness (Huff Lonergan et al., 2010). So the objective of this study was to evaluate the effects of RC on beef quality and to identify the effects of RC on the degradation of the major cytoskeletal proteins in Chinese Yellow crossbred bulls. Meanwhile, it was also investigated whether ES can alleviate the potential negative effects of RC on beef tenderness.
Twenty Chinese Yellow crossbred bulls (Yan-bian× Simmentals) aged 18 months were selected, randomly divided into two groups and slaughtered. The mean slaughter weight was 629.0±50.3 kg. One group was subjected to electrical stimulation (ES: 42 V, 50 Hz, 0.7 A for 40 s) with a commercial ES unit (EST-608, Freund, Germany) immediately after bleeding. The stimulation was applied via the neck region of the carcass, using the rail as the earth. The other group that was not subjected to ES was referred to as the non-stimulated control (NS). After the split process, rapid chilling (RC: −14±1°C for 2 h, air velocity of 3 m/s, then transfer into conventional chilling to 24 h postmortem) was applied to the left half of the carcasses, and conventional chilling (CC: 0 to 4°C for 24 h) was applied to the right half of the carcasses.
The experiment was undertaken following the guidelines of the Animal Ethics Committee in Shandong Agricultural University and all experimental procedures were approved by the State Scientific and Technological Commission (China, 19881114).
Samples (10 g) for SDS-PAGE analysis were removed from the M. longissimus at 2 h (between the 12th and 13th rib interface) postmortem by an expert butcher. After 24 h in the conditioning room, the carcasses were transferred to the cutting room, and the M. longissimus were excised from the carcasses. A series of small samples weighing approximately 10 g were prepared for SDS-PAGE analysis, and 2.54-cm-thick steaks were removed for tenderness determinations, which were vacuum packed and stored at 2±2°C until 7 d postmortem. All samples (10 g) collected at 2 h postmortem and on 1 d, 3 d, and 7 d postmortem were frozen in liquid nitrogen until assayed. The steaks used for tenderness determinations were collected on 1 d, 3 d, and 7 d postmortem respectively, and all were frozen immediately at −20°C until assayed.
At 1 d, 3 d and 7 d postmortem, exposed the fresh meat cut surfaces to the air for 20 min, then the color of the lean tissue was objectively evaluated (X-Rite SP62 Portable Sphere Spectrophotometer, USA) by recording CIE L*, a*, and b* value at three locations across the exposed surface, avoiding areas of clearly visible connective and adipose tissues with care.
Shear force measurements were carried out according to the procedures described by Luo et al. (2008). Briefly, core samples (1.27 cm diameter) were removed from each cooked steak (6 cores/steak), which were parallel to fiber orientation, and then the WBSF was measured, expressed in Newton (N).
Muscle proteins were prepared from M. longissimus at 2 h, 1 d, 3 d, and 7 d postmortem muscle. Approximately 0.4 g of minced muscles and 4 ml of extract buffer (EB: 2% SDS, 10 mmol/L Na3PO4, pH 7.0) were ground using a mortar and pestle and then filtered to remove the large tissues. The protein concentration was determined using the biuret assay. The muscle proteins were stored at −30°C before use.
SDS-PAGE and Western blotting were conducted according to a modification of the method described by Sun et al. (2008). SDS-PAGE was performed using an EPS-301 (Amersham, USA). Two gel systems (16 cm high×16 cm wide×1.5 mm thick) consisting of 12.5% separating gel (acrylamide: N, N′-methylenebisacrylamide = 36.5:1, wt/wt) and 5% stacking gel (acrylamide: N, N′-methylenebisacrylamide = 36.5:1, wt/wt) were used for the protein separation. The running buffer used in both the upper and lower buffer chambers consisted of 25 mM Tris, 192 mM glycine, and 0.l% SDS. The gel was run at 40 mA for 4 h to detect postmortem changes in desmin and troponin-T.
After performing the SDS-PAGE, proteins were transferred from the separating gel to a polyvinylidene fluoride (PVDF) membrane (Immobilon-P, Millipore Corporation, CHELMSFORD, MA, USA) using EPS2A200 (Amersham, USA). The transfer buffer (15% methanol, 20 mM Tris, and 192 mM glycine) was used for the detection of desmin and troponin-T. The electric current used is the membrane area multiplied by 0.8, with a constant current transfer of 70 min. After the transfer, the membranes were blocked with 5% (wt/vol) skim milk powder in TTBS solution (30 mM Tris, pH 7.5, 500 mM NaCl, 0.05% Tween-20) for 2 h at room temperature with gentle rocking, and then washed three times with TTBS solution for 10 min at room temperature. Monoclonal antibodies (Sigma Chemical Co., USA) against desmin (clone DE-U-10) and troponin-T (clone JLT-12) were used as the primary antibodies. Each membrane was incubated with a primary antibody in 5% skim milk powder in TTBS solution for 2.5 h at room temperature and washed three times in TTBS for 10 min each time. Incubation with the secondary antibody (1:4,000, goat anti-mouse IgG alkaline phosphatase-conjugated secondary antibody, Sigma Chemical Co., USA) in TTBS was done for 2 h at room temperature, and the membrane was washed three times with TTBS for 10 min each time. Then the membrane was rinsed with an alkaline phosphatase buffer (0.1 M Tris, pH 9.5, 0.1 M NaCl, 50 mM MgCl2, 1 mM ZnCl2) for 5 min at 25°C. The bound antibodies were visualized by incubating the membranes with the alkaline phosphatase substrate BCIP/NBT, and the membrane was rinsed with distilled water to stop reaction.
The effects of ES and RC on meat color and shear force value were evaluated using the analysis of variance. The means of variable among different treatments were compared using the Student-Newman-Keuls test (p<0.05). All the analyses were performed by SAS 9.0. Figure 1 was fitted to the data (mean value±STD of each treatment) using the SigmaPlot computer package.
In the present study, meat color was not deteriorated by RC (p<0.05), but ES increased L* value at 1 d, 3 d and 7 d (p>0.05) (Table 1). So it can be concluded that RC at −15°C, 2 h had no detrimental effect on meat color. ES could result in brighter beef.
In contrast to the present study, Janz et al. (2001) reported that RC resulted in a darker bison meat color. The reason might be the different breeding or the different animal situations, for example, the different rate of carcass fat coverage. Similar to the present ES results, some literature showed that ES has significant effect on L*, resulting in brighter meat color that persisted until 6 days, and they proposed that ES could be a color enhancing treatment while applied with RC (Janz et al., 2001; Aalhus et al., 1994). The increased free water increases reflectance, which accounts in part for the brighter appearance of the meat.
Statistical results showed that RC increased shear force values at 1 d, 3 d postmortem (p<0.05), while there was no significant differences at 7 d postmortem (p>0.05). However, ES decreased shear force at 1 d, 3 d and 7 d (p<0.05). The shear force values of the four treatments (Figure 1) all decreased with postmortem aging (from 1 d to 7 d postmortem), and the average shear force values of the four group, which are conventionally chilled and electrically stimulated (CC&ES), rapidly chilled and electrically stimulated (RC&ES), conventionally chilled and not electrically stimulated (CC&NS), rapidly chilled and electrically stimulated (RC&NS) were decreased by 38%, 42%, 21%, 24%, respectively. So it can be concluded that RC decreased beef tenderness at early postmortem, and ES not only reduce the detrimental effect of RC, but further enhanced beef tenderness.
Rapid chilling can improve efficiency and facilitate price competition (McGinnisa et al., 1994; Joseph, 1996; Aalhus et al., 2002) and was evaluated by many companies, but its effect on tenderness has not reached a consistent conclusion. The effect of RC on shear force in this study was similar to the research made by White et al. (2006b), it was that RC had no permanent effect on tenderness and the detriment effect of RC could be overcome by postmortem aging.
In the present study, the result agrees with these reports that ES improves beef tenderness (Olsson et al., 1994; Polidori et al., 1999; White et al., 2006a). However, other studies have reported that the application of ES had no significant effect on beef tenderness (Hildrum et al., 1999). These conflicting results may be explained by differences in the status of the animals and the different stimulation methods.
The rate of desmin degradation was affected by the chilling process and ES during postmortem storage (Figure 2). Desmin from all four samples was degraded at different degradation rates during the postmortem aging process.
At 2 h postmortem, the intact desmin band (55-kDa) was not degraded, and there was no critical difference between ES (lane 1) and NS (lane 2). At 1 d postmortem, the desmin began to degrade in CC&ES (lane 3) and CC&NS (lane 5), and degrade faster in the CC&ES (lane 3) sample. However, there was no detectable degradation in RC treated samples (lane 4, 6) at 1 d postmortem. Therefore, it is clear that RC delayed desmin degradation and ES increased it. At 3 d postmortem, desmin showed further degradation in CC&ES (lane 7) and CC&NS (lane 9); however, it seems that there were no changes in RC-treated samples (lane 8, 10) at 3 d postmortem compared with that (lane 4, 6) at 1 d postmortem. At 7 d postmortem, two light degradation bands migrating at approximately 50-kDa (band a) and 41-kDa (band c) appeared, and two dense degradation bands at approximately 46-kDa (band b) and 40-kDa (band d) appeared separately.
Desmin is one of the transversely running elements that link adjacent myofibrils together, and its degradation causes the loss of transversal alignment of the sarcomeres (Rhee et al., 2000). Thus the degradation of desmin can possibly affect the development of tenderness (Ho et al., 1996; Huff Lonergan et al., 2010). Ho et al. (1996) recognized a 38-kDa band in the bovine longissimus muscle aged 7 d by the antibody that was consistently more heavily labeled in the ES samples at 7 d, 14 d, and 28 d postmortem than that in the NS samples. The 38-kDa band is in all probability the 40-kDa band in the present study. In contrast to Ho et al. (1996), it seems that ES had limited effect on desmin that has been aged for 7 d, which conflicted to the results of shear force. This result indicated that mechanical disruption may be a factor associated with improved tenderness in electrically stimulated muscle (Ho et al., 1997).
At 2 h postmortem, the degradation of troponin-T and the appearance of the 30-kDa degradation band (band e) were detected (lane 1, 2) by Western blotting (Figure 3). At 1 d postmortem, the density of the 30-kDa band in the CC& ES sample (lane 3) increased faster than the other three samples (lane 4, 5, 6), and the RC treatment clearly delayed the degradation of troponin-T. At 3 d postmortem, the density of the 30-kDa band increased compared with that at 1 d, especially in RC-treated samples (lane 8, 10). However, the treatment with RC delayed the degradation of troponin-T compared with the treatment with CC both in ES and NS samples at 3 d postmortem (compare lane 7 with lane 8, lane 9 with lane 10, Figure 3). Four degradation bands appeared at approximately 28-kDa (band f), 24-kDa (band g), 23-kDa (band h), and 22-kDa (band i) at 3 d postmortem, and RC treatment decreased the density of degradation bands in both ES and NS samples (compare lane 7 with lane 8, lane 9 with lane 10, Figure 3).
At 7 d postmortem, the effects of both RC and ES treatments on the degradation of troponin-T decreased and the density of the 30-kDa band seemed similar. The 23-kDa band was degraded into two bands, and the density of the 22-kDa band became much denser. Additionally, two other dense bands that were approximately 20-kDa (band j), 16-kDa (band k) appeared. The density of 22-kDa (band i), 20-kDa (band j) and 16-kDa (band k) degradation bands were CC&ES>CC&NS>RC&ES>RC&NS. The results indicated that RC delayed the rate of proteolysis, while ES accelerated it. So it can be concluded that if RC processing is applied, ES combination is recommended.
Huff Lonergan et al. (1996) also found that increased postmortem time was associated with the appearance of the two major bands of approximately 30-kDa and 28-kDa, which were labeled with monoclonal antibodies of troponin-T. As the results of troponin-T degradation, the appearance of the 30-kDa component was proposed to be an indicator for proteolysis (Macbride and Parrish, 1977; Olson and Parrish, 1977; Ho et al., 1994; White et al., 2006a). Huff Lonergan et al. (2010) also showed that the appearance of the 30-kDa and 28-kDa bands in the myofibril was strongly related to the shear force. Olson et al. (1977) showed that the intensity of the 30-kDa component corresponds to the tenderness level of the steaks and proposed that the intensity of the 30-kDa component is a useful index of tenderness. In the present study, the density of the 30-kDa band was delayed by the RC treatment and increased by the ES treatment until 3 d postmortem. Previous researches also concluded that low temperature conditions delay the degradation of troponin-T (Hwang et al., 2004; White et al., 2006b). However, it appeared that rapidly and slowly chilled muscles underwent proteolysis to the same extent at 21 d postmortem, and this study revealed that the early postmortem conditions influenced the rate but not the extent of proteolysis during ageing at 2°C for 21 days (White et al., 2006b; White et al., 2006c). Therefore, RC and ES treatments possibly play an important role in the degradation of troponin-T and influence tenderness especially at the early postmortem stage. However, in contrast to the present study, White et al. (2006a) found that ES did not appear to have a positive effect on proteolysis as monitored by qualitative SDS-PAGE. Some literatures also reported that ES increased the degradation of desmin and troponin-T in Angus×Jersey longissimus muscle, but had no effect on degradation of desmin and troponin-T in Bos indicus Crossbred Cattle (Ho et al., 1996; Ho et al., 1997). These differences may due to the different detect methods and breeds of cattle.
Chilling rate is quite critical because too slow or too fast chilling of beef can result in an inferior meat quality (Van Moeseke et al., 2001). The early postmortem chilling rate influences beef tenderness by changing sarcomere length (Locker and Hagyard, 1963; Marsh and Leet, 1966; Wheeler and Koomaraie, 1999; Hwang et al., 2004; White et al., 2006c), affecting enzyme activity (Joseph, 1996; Hwang et al., 2004) and physical restraint (Joseph, 1996; Van Moeseke et al., 2001).
Commercial chilling regimes have been designed to try to avoid carcass temperatures below 10°C within the first 10 h postmortem to prevent toughening (Joseph, 1996; Aalhus et al., 2002). Also several studies proposed that meat reaching rigor mortis at 15°C result in optimum tenderization (Geesink et al., 2000; Hwang et al., 2003). Aalhus et al. (2002) found that carcass chilling at −20°C tended to increase shear force values in the longissimus thoracis at 1 d postmortem and after 21 d of postmortem aging, but there were no significant effect on semimembranosus; Carcass chilling at −35°C for 10 h could produce more tender beef at 6 d postmortem and the differences disappeared with extended postmortem aging to 21 d (Aalhus et al., 2002). And other reports also indicated that very fast chilling could result in improvements in tenderness (Davey and Garnett, 1980; Bowling et al., 1987; Sheridan, 1990). Proteolysis and mechanical restraint brought on by surface freezing are considered to be the reasons to produce tender beef (Joseph, 1996; Aalhus et al., 2002).
Considering all literatures cited above, we believe that if factors, which can improve tenderness, play a dominant role and can overcome the detriment effect, the tenderness will be improved. But whether a chilling rate will increase or decrease the meat tenderness and the mechanism of effects on tenderness need further research. Furthermore, animal situation (genotypic variations, pre-slaughter transport and handling, carcass weight, back fat, anatomical muscle site) should be considered besides chilling rate when investigating beef tenderness.
In the present study, troponin-T began to degrade and the 30-kDa polypeptide began to appear at 2 h postmortem. In support, Koohmaraie et al. (1987) stated that tenderization begins soon after exsanguination. Although Wheeler and Koohmaraie (1994) proposed that a sufficient amount of proteolysis may have occurred by 24 h postmortem to affect tenderness, they did not detect the 28 to 32 kDa degradation products until 12 h postmortem and did not detect any desmin or troponin-T degradation until 24 h postmortem by SDS-PAGE. Compared to SDS-PAGE, the western blotting antibody detection method might be more sensitive. Thus, using western bolting methods in this study can detect the degradation of troponin-T in 2 h postmortem.
Western blotting detected troponin-T degradation at 2 h postmortem, but did not detect desmin degradation until 1 d postmortem. Therefore, the degradation of troponin-T was observed to be earlier than the degradation of desmin, and it may be concluded that troponin-T can be used as a better indicator for the start of tenderization process in the early stage. In the present study, there are some small peptides degradation bands appeared and these peptides may also be related to shear force, similar to the 30 kDa polypeptide. Furthermore, tenderness improved along with the cytoskeletal protein degradation, but they are not completely consistent. So the beef postmortem aging may contain some other mechanisms and need further research.
This study reveals that cytoskeletal proteins can be degraded during postmortem aging, and the results agree with previous studies which showed that beef tenderness increases with extended postmortem aging time. Interestingly, in contrast to the highest shear force at 24 h postmortem (Wheeler et al., 1994), the extent of desmin and troponin-T degradation at 24 h postmortem was greater than that at 2 h postmortem, so it seems that the tenderness was affected by other factors at early postmortem. Some studies revealed that tenderness varies depending upon the rigor shortening or the degradation of muscle proteins by proteases (Miller et al., 2001; Savell et al., 2005; White et al., 2006a). Some studies indicated that the sarcomere length determines tenderness before extensive proteolysis (Wheeler et al., 1994; Hwang et al., 2004). So this study confirmed the tenderness at 24 h postmortem is not determined by cytoskeletal proteins degradation, and it is assumed that other factors such as sarcomere length are the major determinants, just as the above reports made by Wheeler et al. (1994) and Hwang et al. (2004).
RC decreases beef tenderness at 1 d, 3 d postmortem, but not deteriorates meat color. ES can improve meat lightness and beef tenderness at 1 d, 3 d, 7 d postmortem. The degradation rate of desmin and troponin-T were delayed by RC and increased by ES. Combination of RC and ES can not only accelerate postmortem aging rate, but further improve beef tenderness. So combination of RC and ES was recommended to increase production rate and improve beef quality.
ACKNOWLEDGEMENTS
The work was supported by the CARS-38 from Ministry of Agriculture of the People’s Republic of China and a national non-profit project (industry) (200903012).
Figure 1
Shear force of M. longissimus at 1, 3, and 7 d post-mortem with different treatments. CC&ES: carcasses which were conventionally chilled and electrically stimulated after slaughter; RC&ES: carcasses which were rapidly chilled and electrically stimulated after slaughter; CC&NS: carcasses which were conventionally chilled and not electrically stimulated after slaughter; RC&NS: carcasses which were rapidly chilled and not electrically stimulated after slaughter.

Figure 2
Western blots prepared from 12.5% gel of samples during postmortem storage and labeled with desmin mono-colonal antibodies. CC&ES: carcasses which were conventionally chilled and electrically stimulated after slaughter; RC&ES: carcasses which were rapidly chilled and electrically stimulated after slaughter; CC&NS: carcasses which were conventionally chilled and not electrically stimulated after slaughter; RC&NS: carcasses which were rapidly chilled and not electrically stimulated after slaughter. Lane 1 show ES; lane 2 show NS; lanes 3, 7, and 11 show CC&ES; lanes 4, 8, and 12 show RC&ES; lanes 5, 9, and 13 show CC&NS; lanes 6, 10, and 14 show RC&NS. The letters a, b, c, d mark the key degraded bands 50-kDa (band a), 46-kDa (band b), 41-kDa (band c) and 40-kDa (band d) in the figure.
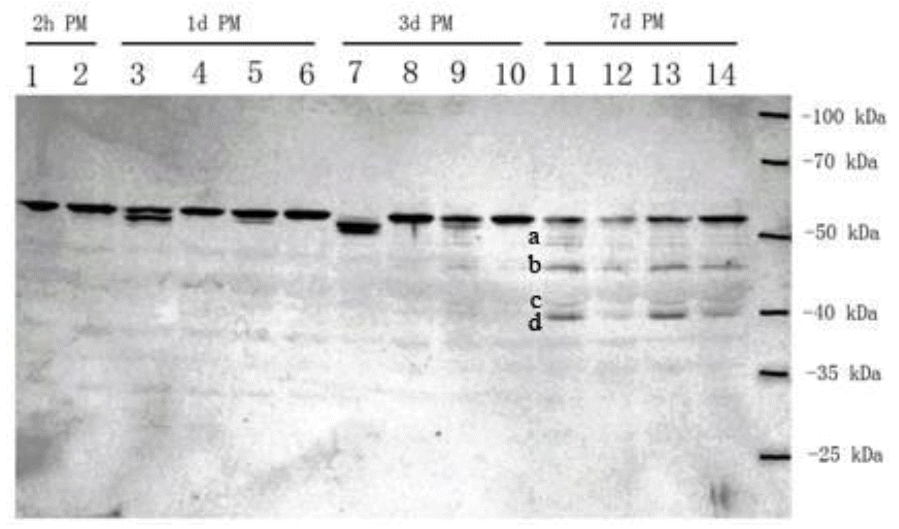
Figure 3
Western blots prepared from 12.5% gel of samples during postmortem storage and labeled with troponin-T mono-colonal antibodies. CC&ES: carcasses which were conventionally chilled and electrically stimulated after slaughter; RC&ES: carcasses which were rapidly chilled and electrically stimulated after slaughter; CC&NS: carcasses which were conventionally chilled and not electrically stimulated after slaughter; RC&NS: carcasses which were rapidly chilled and not electrically stimulated after slaughter. Lane 1 show ES; lane 2 show NS; lanes 3, 7, and 11 show CC&ES; lanes 4, 8, and 12 show RC&ES; lanes 5, 9, and 13 show CC&NS; lanes 6, 10, and 14 show RC&NS. The letters e, f, g, h, I, j, k mark the key degraded bands 30-kDa (band e), 28-kDa (band f), 24-kDa (band g), 23-kDa (band h), 22-kDa (band i) and 20-kDa (band j) 16-kDa (band k) in the figure.

Table 1
Effects of different treatments on meat color of M. longissimus at 1, 3, and 7 d postmortem
Different letters in the same row indicate a significant difference (p<0.05). CC&ES: carcasses which were conventionally chilled and electrically stimulated after slaughter; RC&ES: carcasses which were rapidly chilled and electrically stimulated after slaughter; CC&NS: carcasses which were conventionally chilled and not electrically stimulated after slaughter; RC&NS: carcasses which were rapidly chilled and not electrically stimulated after slaughter.
REFERENCES
Aalhus JL, Jones SDM, Lutz S, Best DR, Robertson WM. 1994. The efficacy of high and low voltage electrical stimulation under different chilling regimes. Can J Anim Sci 74:433–442.

Aalhus JL, Robertson WM, Dugan MER, Best DR. 2002. Very fast chilling of beef carcasses. Can J Anim Sci 82:59–67.

Bowling RA, Dutson TR, Smith GC, Savell JW. 1987. Effects of cryogenic chilling on beef carcass grade, shrinkage and palatability characteristics. Meat Sci 21:67–72.


Davey CL, Garnett KJ. 1980. Rapid freezing, frozen storage and the tenderness of lamb. Meat Sci 4:319–322.


Geesink GH, Bekhit AD, Bickerstaffe R. 2000. Rigor temperature and meat quality characteristics of lamb longissimus muscle. J Anim Sci 78:2842–2848.


Hildrum KI, Solvang M, Nilsen BN, Frøystein T, Berg J. 1999. Combined effects of chilling rate, low voltage electrical stimulation and freezing on sensory properties of bovine M. longissimus dorsi. Meat Sci 52:1–7.


Ho CY, Stromer MH, Robson RM. 1994. Identification of the 30 kDa polypeptide in postmortem skeletal muscle as a degradation product of troponin-T. Biochimie 76:369–375.


Ho CY, Stromer MH, Robson RM. 1996. Effect of electrical stimulation on postmortem titin, nebulin, desmin, and troponin-T degradation and ultrastructural changes in bovine longissimus muscle. J Anim Sci 74:1563–1575.


Ho CY, Stromer MH, Rouse G, Robson RM. 1997. Effects of electrical stimulation and postmortem storage on changes in titin, nebulin, desmin, troponin-T, and muscle ultrastructure in Bos indicus crossbred cattle. J Anim Sci 75:366–376.


Huff Lonergan E, Mitsuhashi T, Parrish FC, Robson RM. 1996. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blotting comparisons of purified myofibrils and whole muscle preparations for evaluating titin and nebulin in postmortem bovine muscle. J Anim Sci 74:779–785.


Huff Lonergan E, Zhang W, Lonergan SM. 2010. Biochemistry of postmortem muscle-Lessons on mechanisms of meat tenderization. Meat Sci 86:184–195.


Hwang IH, Park BY, Cho SH, Lee JM. 2004. Effects of muscle shortening and proteolysis on Warner-Bratzler shear force in beef longissimus and semitendinosus. Meat Sci 68:497–505.


Janz JAM, Aalhus JL, Price MA. 2001. Blast chilling and low voltage electrical stimulation influences on bison (Bison bison bison) meat quality. Meat Sci 57:403–411.


Joseph RL. 1996. Very fast chilling of beef and tenderness-a report from an EU concerted action. Meat Sci 43:Supplement 1217–227.

Koohmaraie M, Seidemann SC, Schollmeyer JE, Dutson TR, Crouse JD. 1987. Effect of post-mortem storage on Ca++-dependent proteases, their inhibitor and myofibril fragmentation. Meat Sci 19:187–196.


Li CB, Chen YJ, Xu XL, Huang M, Hu TJ, Zhou GH. 2006. Effects of low-voltage electrical stimulation and rapid chilling on meat quality characteristics of Chinese Yellow crossbred bulls. Meat Sci 72:9–17.

Luo X, Zhu Y, Zhou GH. 2008. Electron microscopy of contractile bands in low voltage electrical stimulation beef. Meat Sci 80:948–951.


Macbride MA, Parrish FC. 1977. The 30,000-dalton component of tender bovine longissimus muscle. J Food Sci 42:1627–1629.

Marsh BB, Leet NG. 1966. Studies in Meat Tenderness. III. The effects of cold shortening on tenderness. J Food Sci 31:450–459.

McGinnisa DS, Aalhusa JL, Chabota B, Gariépyb C, Jonesa SDM. 1994. A modified hot processing strategy for beef: reduced electrical energy consumption in carcass chilling. Food Res Int 27:527–535.

Miller MF, Carr MA, Ramsey CB, Crockett KL, Hoover LC. 2001. Consumer thresholds for establishing the value of beef tenderness. J Anim Sci 79:3062–3068.


Olson DG, Parrish FC. 1977. Relationship of myofibril fragmentation index to measures of beef steak tenderness. J Food Sci 42:506–509.

Olsson U, Hertzman C, Tornburg E. 1994. The influence of low temperature, type of muscle and electrical stimulation on the course of rigor mortis, ageing and tenderness of beef muscles. Meat Sci 37:115–131.


Polidori P, Lee S, Kauffman RG, Marsh BB. 1999. Low voltage electrical stimulation of lamb carcasses: effects on meat quality. Meat Sci 53:179–182.


Rhee MS, Ryu YC, Imm JY, Kim BC. 2000. Combination of low voltage electrical stimulation and early postmortem temperature conditioning on degradation of myofibrillar proteins in Korean native cattle (Hanwoo). Meat Sci 55:391–396.


Sun QL, Luo X, Mao YW, Zhang YM. 2008. Effect of electrical stimulation and delay chilling on the degradation of the Troponin-T from myofibrillar in the post slaughter beef. Transactions of the CSAE 24:262–266.
Van Moeseke W, De Smet S, Claeys E, Demeyer D. 2001. Very fast chilling of beef: effects on meat quality. Meat Sci 59:31–37.


Wheeler TL, Koohmaraie M. 1994. Prerigor and postrigor changes in tenderness of ovine longissimus muscle. J Anim Sci 72:1232–1238.


Wheeler TD, Koomaraie M. 1999. The extent of proteolysis is independent of sarcomere length in lamb longissimus and psoas major. J Anim Sci 77:2444–2451.


White A, O’Sullivan A, Troy DJ, O’Neill EE. 2006a. Effects of electrical stimulation, chilling temperature and hot-boning on the tenderness of bovine muscles. Meat Sci 73:196–203.


- TOOLS





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





