 |
 |
Abstract
Compensatory growth of grower olive flounder (Paralichthys olivaceus) was determined at suboptimal temperature (13.0±1.9°C). Fifteen fish averaging 201.1 g per tank were distributed into 18 of 300 L flow-through tanks. Six treatments were prepared in triplicate: fish were hand-fed with an extruded pellet to apparent satiation once a day for 16 weeks (16 WF); and the other five groups of fish were hand-fed for 15, 14, 13, 12 and 10 weeks after 1-, 2-, 3-, 4- and 6-week feed deprivation, referred to as 15 WF, 14 WF, 13 WF, 12 WF and 10 WF, respectively. A linear relationship between body weight of fish and feed deprivation was observed: Y (Body weight of fish) = −1.81X (Weeks of feed deprivation)+201.07, R2 = 0.83. Weight gain of grower olive flounder in 15 WF, 14 WF, 13 WF and 12 WF treatments was comparable to that of fish in 16 WF treatment, but lower than that of fish in 10 WF treatment. Specific growth rate of fish in 15 WF treatment was higher than that of fish in 16 WF, 14 WF and 10 WF treatments. Feed consumption of fish was not affected by feeding regime. Feed and protein efficiency ratios of fish in 15 WF treatment were higher than those of fish in 13 WF, 12 WF and 10 WF treatments. Grower olive flounder could achieve full compensatory growth when fish were daily fed for 12 weeks after 4-week feed deprivation at suboptimal temperature.
Olive flounder (Paralichthys olivaceus) is one of the most commercially important marine fish species for aquaculture in Eastern Asia including Korea, Japan and China, and its optimum temperature conditions was known to range 20–25°C (Iwata et al., 1994). However, water temperature can rise above 30°C in the summer and fall below 10°C in the winter in wild. Therefore, this fish species seems to tolerate a wide range of temperature.
Compensatory growth of fish is rapid or faster than normal growth rate of fish resulting from refeeding after fasting. Achieving compensatory growth of fish can have the several advantages for aquaculture such as saving feed and labor costs, reducing water pollution during starvation, and improving feeding activity and feed efficiency shortly after refeeding. Huang et al. (2008) reported that juvenile olive flounder averaging 12 g subjected to low temperature of 8.5°C for 10 days and followed by being grown at an optimum temperature of 22°C for the next 30 days achieved full compensatory growth. In addition, juvenile olive flounder averaging 53.9 g and 16 g achieved full compensatory growth after the 2-week feed deprivation at mean temperatures of 15°C and 23.6°C in the 8-week trials (Cho, 2005; Cho et al., 2006).
However, compensatory growth responses could vary depending on several factors such as fish species (Jobling et al., 1994; Wang et al., 2000), fish size (Bilton and Robins, 1973), dietary nutrient composition (Cho and Heo, 2010), duration of feeding trial (Rueda et al., 1998; Heide et al., 2006), feeding regime (Chatakondi and Yant, 2001; Zhu et al., 2004; Oh et al., 2007; Cho and Cho, 2009) and social factors (Jobling and Koskela, 1996; Hayward et al., 1997, 2000). Furthermore, Ali et al. (2003) indicated that partial, full and overcompensation could be evoked in fish although overcompensation had only been observed when cycles of deprivation and satiation feeding had been imposed.
Generally speaking, grower olive flounder must overwinter to grow up to highly valuable marketing size at fish farm. To date, there have been only few studies with grower olive flounder (Kim et al., 2009) compared to studies with juvenile fish (Iwata et al., 1994; Cho, 2005; Huang et al., 2008; Cho and Heo, 2010). In addition, no study on compensatory growth of grower olive flounder has been performed yet. In this study, therefore, compensatory growth of grower olive flounder with different feeding regime was determined at suboptimal temperature.
Grower (ca. 200 g) olive flounder were purchased from a private farm and acclimated to the experimental conditions for 4 weeks. During the acclimation period, fish were fed with the commercial extruded pellet (EP) once a day as much as they voluntarily eat which was about 0.2–0.5% total biomass. Fifteen fish (an initial body weight of fish: 201.1±0.2 g) per tank were randomly chosen and distributed into 18 of 300 L flow-through tanks (water volume 250 L). The water source was the sand-filtered natural seawater and aeration supplied to each tank. The flow rate of water into each tank was 8.6 L/min. Water temperature ranged from 9.8°C to 16.5°C (mean±SD: 13.0±1.9°C) and photoperiod left natural condition.
Six treatments were prepared in triplicate: fish were hand-fed with the EP (Suhyup Feed Co. Ltd., Korea) containing 51.6% crude protein and 14.9% crude lipid to apparent satiation once a day (09:00), seven days a week, for 16 week (16 WF), which was used as a control group; and five other groups of fish were hand-fed with the EP to apparent satiation once a day for 15, 14, 13, 12 and 10 weeks after 1-, 2-, 3-, 4- and 6-week feed deprivation, referred to as 15 WF, 14 WF, 13 WF, 12 WF and 10 WF, respectively. Fish from each tank were weighed to measure reduction in body weight of fish a day before feeding according to designated feeding schedule. At the end of 16-week trial, fish from each tank were weighed after being starved for a day.
Following final weighing five fish from each tank were sampled. Total fish length (mm) and fish weight (g) calculated by the weight of biomass in tank divided by number of fish was measured. Liver was obtained from samples and weighed. Following indices were calculated; CF = body weight (g)/total length (cm)3 and HSI = liver weight/body weight. Other criteria measured were as follows; specific growth rate (SGR) = (Ln final weight of fish-Ln initial weight of fish)×100/days of feeding trial, feed consumption (g/fish) = total feed consumption/number of fish, feed efficiency ratio (FER) = weight gain of fish/feed consumed and protein efficiency ratio (PER) = weight gain of fish/protein consumed.
Three fish at the beginning and from each tank at the end of the study were sampled for proximate analysis. Fish were dissected and the whole body excluding the liver and liver of fish were separated. The whole body of fish excluding liver and the liver were separately homogenized before proximate analysis. Crude protein content determined using Kjeldahl method (Kjeltec 2100 Distillation Unit, Foss Tecator, Hoganas, Sweden), lipid content determined using ether-extraction method, moisture content determined by drying sample in a dry oven at 105°C for 24 h, fiber content determined using automatic analyzer (Fibertec, Tecator, Hoganas, Sweden) and ash content determined using muffle furnace at 550°C for 4 h, all methods were according to standard AOAC (1990).
One-way ANOVA and least significant difference (Fisher’s LSD) test were used to analyze the significance (p<0.05) of the difference among the means of treatments. Regression analysis for weight loss of olive flounder during starvation was conducted using SAS Version 9.1 (SAS Institute, Cary, NC, USA). All percentage data were transformed to arcsine values prior to analysis.
A linear relationship between body weight of grower olive flounder (Y) and weeks of feed deprivation (X) was observed: Y = −1.81X+201.07, R2 = 0.83 (Figure 1). A higher negative slope (−1.81) relationship between body weight of grower olive flounder and weeks of feed deprivation indicates that a greater weight loss per week of feed deprivation than that observed in juvenile olive flounder (slope = −0.81 at 15°C and slope = −0.57 at 23.6°C (Cho, 2005; Cho et al., 2006), agreeing with Adelman et al. (1955)’s study showing that larger brook trout (Salvelinus fontinalis) lost more body weight than smaller fish when starved for 6 months.
Survival over the 16 week experimental period ranged from 91% to 100% was not significantly (p>0.1) different among treatments (Table 1). However, weight gain of grower olive flounder in 15 WF, 14 WF, 13 WF and 12 WF treatments was comparable to that of fish in 16 WF treatment, but significantly (p<0.02) lower than that of fish in 10 WF treatment. This indicated that grower olive flounder could achieve full compensatory growth after 4-week feed deprivation at suboptimal temperature in this study. Similarly, channel catfish (averaging 22 g and 420 g) partially fed achieved same weight gain as fish continuously fed when fish were continuously fed, partially or non-fed throughout the overwintering period from December to April at earthen ponds (Kim and Lovell, 1995). When juvenile olive flounder were subjected to a range of temperature treatments (8.5, 13.0, 17.5, 22.0 and 26.5°C) for 10 days, even the fish held at 8.5°C achieved full compensation when subsequently reared at 22.0°C for 30 days (Huang et al., 2008). The results of Cho (2005), Cho et al. (2006) and Huang et al. (2008) and the present study probably indicated that eurythermal fish like the olive flounder could display compensatory growth over a range of temperatures.
The mean daily specific growth rate (SGR) in the 15 WF treatment was significantly (p<0.01) higher than that of fish in 16 WF, 14 WF and 10 WF treatments, but not significantly (p>0.05) different from that of fish in 13 WF and 12 WF treatments in this study. The mean daily SGR of fish in 15 WF, 14 WF, 13 WF and 12 WF treatments was also higher than that of fish in 10 WF treatment. Oliver flounder over 150–200 g are usually fed to satiation no more than once a day at fish farm for easy management and cost effective production. Since the daily mean SGR (0.16%/d) of grower olive flounder in this study was comparable to that (0.20%/d) of fish grown from 270 to 350 g in Kim et al. (2009)’s study, in which fish were fed with the commercial EP (52.3% crude protein and 12.8 crude lipid) to satiation once a day at mean temperature of 12°C for 15 weeks, growth of fish seemed to be well achieved in this study. Recently, Kim et al. (2010) showed that SGR of grower olive flounder averaging 255 g ranged 0.18–0.56%/d when fish were fed with the diets containing various protein (40, 45 and 50%) and lipid (7 and 14%) levels to satiation twice a day at 15.5°C for 14 weeks. In addition, Iwata et al. (1994) reported SGR values of 0.18 and 0.71%/d for olive flounder averaging 176 g when fish were fed with the commercial diet (60% crude protein and 14% crude lipid) to satiation twice a day at 10 and 15°C for 35 days, respectively.
Feed consumption (g/fish) of grower olive flounder was not significantly (p>0.08) affected by feeding regime (Table 2). No difference in feed consumption of fish in this study probably supported full compensatory growth of fish. This agreed with other studies (Rueda et al., 1998; Gaylord and Gatlin, 2000; Wang et al., 2000; Tian and Qin, 2004; Cho, 2005; Cho et al., 2006; Oh et al., 2007) showing that hyperphagia was observed in fish achieving full compensatory growth. Although feed consumption of olive flounder in 10 WF treatment was almost same as in 16 WF treatment, lower weight gain of fish was observed in the latter.
However, feed and protein efficiency ratios of fish in 15 WF treatment were significantly (p<0.001) higher than those of fish in 13 WF, 12 WF and 10 WF treatments, but not significantly (p>0.05) different from those of fish in 16 WF and 14 WF treatments in this study. Feed and protein efficiency ratios of fish in 16 WF, 14 WF, 13 WF and 12 WF treatments were also higher than those of fish in 10 WF treatment, which was lowest. Higher feed and protein efficiency ratios of fish in 16 WF, 15 WF, 14 WF, 13 WF and 12 WF treatments compared to those of fish in 10 WF treatment partially agreed with other studies (Jobling et al., 1994; Gaylord and Gatlin, 2000, 2001; Cho, 2005; Oh et al., 2007; Cho and Heo, 2010) showing that improvement in feed utilization was observed in fish achieving compensatory growth.
Neither CF (p>0.8) nor HSI (p>0.5) of grower olive flounder was different among treatments in this study. Unlike this study, however, HSI was a good index to indicate compensatory growth of channel catfish and olive flounder (Gaylord and Gatlin, 2000; Cho, 2005). In addition, Miglavs and Jobling (1989) showed that the relative sizes of liver and viscera decreased with either feed deprivation for 16 weeks or restricted feeding, which was 10% of feed consumption at satiation feeding for 8 weeks in Arctic charr (Salvelinus aplinus) and increased to levels exceeding those of fish fed continuously to satiation after fish were transferred from restricted to satiation feeding for the following 8 weeks.
Moisture (p>0.7), crude lipid (p>0.1) and ash (p>0.8) content of the whole body excluding liver and moisture (p>0.9) and crude lipid (p>0.1) content of liver of grower olive flounder was not significantly affected by feeding regime (Table 3). However, crude protein content of the whole body excluding liver of fish in the 12 WF treatment was significantly (p<0.01) higher than for fish in the other treatments with the exception of the 16 WF treatment. In addition, crude protein content of the whole body excluding liver for fish in the 16 WF treatment was also higher than for fish in the 13 WF treatment, which had the lowest level. Crude protein content of liver of fish in 15 WF treatment was significantly (p<0.0001) higher than that of liver of fish in all other treatments except for that of the liver of fish in 12 WF treatment. In addition, crude protein content of liver of fish in 16 WF, 14 WF and 13 WF treatments was also higher than that of liver of fish in 10 WF treatment, which was lowest.
When rainbow trout (Oncorhynchus mykiss) and gibel carp (Carassius auratus gibelio) were starved for a defined period of time, body fat decreased, but body moisture and protein increased compared to fish without feed deprivation; however, the fish restored the body fat shortly after satiation feeding (Quinton and Blake, 1990; Xie et al., 2001). This resulted to no difference in body proximate composition of fish with different feeding regimes at the end of the feeding trials (Gaylord and Gatlin, 2000; Xie et al., 2001; Zhu et al., 2004; Cho et al., 2006). Miglavs and Jobling (1989) reported that lipid decreased and water increased in the eviscerated carcass of Arctic charr with feed deprivation; however, liver lipid content of restricted-satiation fish followed by satiation feeding was higher than that of fish fed to satiation without feed deprivation in 16-week feeding trial.
In conclusion, grower olive flounder could achieve full compensatory growth up to 4-week feed deprivation when fish were fed with extruded pellet to apparent satiation once a day for 16 weeks or 15, 14, 13, 12 and 10 weeks after 1-, 2-, 3-, 4- and 6-week feed deprivation at suboptimal temperature.
ACKNOWLEDGMENTS
This research was a part of the project titled “Development of Technologies for Risk Assessment of Marine Living Genetically Modified Organisms” funded by the Ministry of Land, Transport and Maritime Affairs, Korea.
Figure 1
Change in body weight (g/fish) of grower olive flounder with weeks of feed deprivation (n = 3): Y (Body weight of fish) = −1.81X (Weeks of feed deprivation)+201.07, R2 = 0.83).
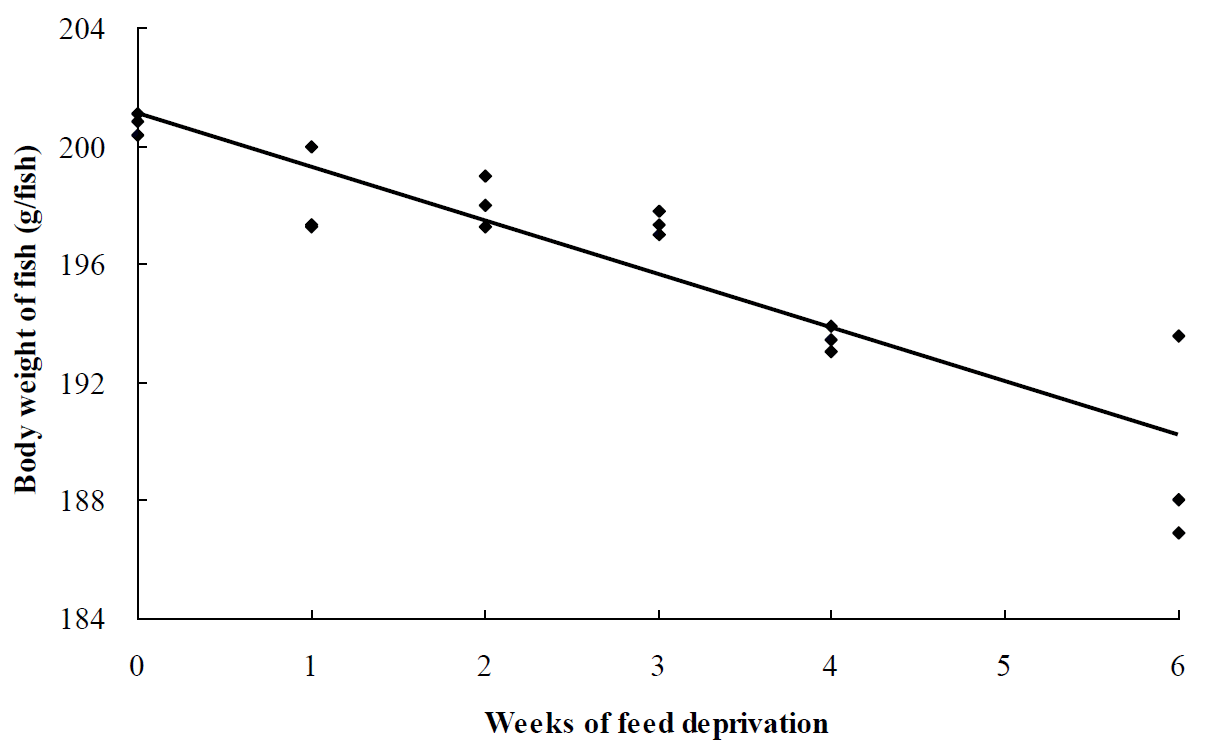
Table 1
Survival (%), weight gain (g/fish) and specific growth rate (SGR) of grower olive flounder fed the extruded pellet with different feeding regime for 16 weeks
| Treatments | Initial weight of fish (g/fish) | Final weight of fish (g/fish) | Survival (%) | Weight gain (g/fish) | SGR1 (%/d) |
|---|---|---|---|---|---|
| 16 WF | 200.78±0.21 | 239.83±4.23 | 97.78±2.22 | 39.05±4.30a | 0.16±0.02bc |
| 15 WF | 201.09±0.18 | 275.38±15.71 | 91.11±4.44 | 74.30±15.53a | 0.28±0.05a |
| 14 WF | 201.36±0.21 | 247.80±11.04 | 100.00±0.00 | 46.44±10.83a | 0.18±0.04b |
| 13 WF | 201.36±0.23 | 247.69±10.26 | 100.00±0.00 | 46.33±10.25a | 0.18±0.04ab |
| 12 WF | 201.29±0.12 | 254.56±2.89 | 100.00±0.00 | 53.27±3.00a | 0.21±0.01ab |
| 10 WF | 201.04±0.27 | 218.56±2.21 | 95.56±2.22 | 17.52±2.26b | 0.07±0.01c |
Table 2
Feed consumption (g/fish), feed efficiency ratio (FER), protein efficiency ratio (PER), condition factor (CF) and hepatosomatic index (HSI) of grower olive flounder fed the extruded pellet with different feeding regime for 16 weeks
| Treatments | Feed consumption | FER1 | PER2 | CF3 | HSI4 |
|---|---|---|---|---|---|
| 16 WF | 47.29±3.47 | 0.82±0.04ab | 1.59±0.08ab | 1.01±0.04 | 1.46±0.13 |
| 15 WF | 81.14±17.79 | 0.92±0.02a | 1.78±0.14a | 0.98±0.03 | 1.76±0.03 |
| 14 WF | 64.27±15.44 | 0.74±0.10abc | 1.43±0.20abc | 0.97±0.11 | 1.46±0.17 |
| 13 WF | 63.38±7.43 | 0.72±0.07bc | 1.39±0.14bc | 1.09±0.09 | 1.52±0.17 |
| 12 WF | 91.39±9.99 | 0.60±0.07c | 1.16±0.14c | 1.13±0.11 | 1.71±0.03 |
| 10 WF | 46.66±6.03 | 0.38±0.03d | 0.73±0.06d | 0.98±0.05 | 1.68±0.18 |
Table 3
Chemical composition (%, wet weight) of the whole body excluding liver and liver of grower olive flounder at the end of the 16-week feeding trial
REFERENCES
Ali M, Nicieza A, Wootton RJ. 2003. Compensatory growth in fishes: a response to growth depression. Fish Fish 4:147–190.

AOAC. 1990. Official methods of analysis. 15th ednAssociation of Official Analytical Chemists; Arlington, Virginia, USA:
Adelman HM, Bingham JL, Maatch JL. 1955. The effect of starvation upon brook trout of three sizes. Progres Fish Cult 17:110–112.

Bilton HT, Robins GL. 1973. The effects of starvation and subsequent feeding on survival and growth of Fulton channel sockeye salmon fry. J Fish Res Board Can 30:1–5.

Chatakondi NG, Yant RD. 2001. Application of compensatory growth to enhance production in channel catfish Ictalurus punctatus. J World Aquac Soc 32:278–285.

Cho SH. 2005. Compensatory growth of juvenile flounder Paralichthys olivaceus L. and changes in biochemical composition and body condition indices during starvation and after refeeding during the winter season. J World Aquac Soc 36:508–514.

Cho YJ, Cho SH. 2009. Compensatory growth of olive flounder, Paralichthys olivaceus, fed the extruded pellet (EP) with different feeding regimes. J World Aquac Soc 40:505–512.

Cho SH, Heo T. 2011. Effect of dietary nutrient composition on compensatory growth of juvenile olive flounder Paralichthys olivaceus using different feeding regimes. Aqacult Nutr 17:90–97.

Cho SH, Lee S, Park BH, Ji S, Lee J, Bae J, Oh S. 2006. Compensatory growth of juvenile olive flounder Paralichthys olivaceus L. and changes in proximate composition and body condition indexes during fasting and after refeeding in summer season. J World Aquac Soc 37:168–174.

Gaylord TG, Gatlin DM. 2000. Assessment of compensatory growth in channel catfish Ictalurus punctatus R. and associated changes in body condition indices. J World Aquac Soc 31:326–336.

Gaylord TG, Gatlin DM. 2001. Dietary protein and energy modifications to maximize compensatory growth of channel catfish (Ictalurus punctatus). Aquaculture 194:337–348.

Hayward RS, Noltie DB, Wang N. 1997. Use of compensatory growth to double hybrid sunfish growth rate. Trans Am Fish Soc 126:316–322.

Hayward RS, Wang N, Noltie DB. 2000. Group holding impedes compensatory growth of hybrid sunfish. Aquaculture 183:299–305.

Heide A, Foss A, Stefansson SO, Mayer I, Norberg B, Roth B, Jenssen MD, Nortvedt R, Imsland AK. 2006. Compensatory growth and fillet crude composition in juvenile Atlantic halibut: Effects of short term starvation periods and subsequent feeding. Aquaculture 261:109–117.

Huang G, Wei L, Zhang X, Gao T. 2008. Compensatory growth of juvenile flounder Paralichthys olivaceus(Temminck & Schelgel) following thermal manipulation. J Fish Biol 72:2534–2542.

Iwata N, Kikuchi K, Honda H, Kiyono M, Kurokura H. 1994. Effects of temperature on the growth of Japanese flounder, Paralichthys olivaceus. Fish Sci 60:527–531.

Jobling M, Meloy OH, Dos Santos J, Christiansen B. 1994. The compensatory growth response of the Atlantic cod: effects of nutritional history. Aquacult Int 2:75–90.

Jobling M, Koskela J. 1996. Interindividual variations in feeding and growth in rainbow trout during restricted feeding and in a subsequent period of compensatory growth. J Fish Biol 49:658–667.

Kim MK, Lovell RT. 1995. Effect of overwinter feeding regimen on body weight, body composition and resistance to Edwardsiella ictaluri in channel catfish, Ictarulus punctatus. Aquaculture 134:237–246.

Kim K, Nam M, Kim K, Lee H, Hur S, Kang YJ, Son MH. 2009. Effects of feeding rate and feeding frequency on growth and body composition of sub-adult flounder Paralichthys olivaceus in suboptimal water temperature. Kor J Fish Aqua Sci 42:262–267.

Kim K, Kang YJ, Lee HM, Kim K, Jang M, Choi S. 2010. Effects of dietary protein and lipid levels on growth and body composition of subadult olive flounder, Paralichthys olivaceus, at a suboptimal water temperature. J World Aquac Soc 41:263–269.

Miglavs I, Jobling M. 1989. The effect of feeding regime on proximate body composition and patterns of energy deposition in juvenile Arctic charr, Salvelinus alpinus. J Fish Biol 35:1–11.

Oh SY, Noh CH, Cho SH. 2007. Effect of restricted feeding regimes on compensatory growth and body composition of red sea bream, Pagrus major. J World Aquac Soc 38:443–449.

Quinton JC, Blake RW. 1990. The effect of feed cycling and ration level on the compensatory growth response in rainbow trout, Oncorhynchus mykiss. J Fish Biol 37:33–41.

Rueda FM, Martinez FJ, Zamora S, Kentouri M, Divanach P. 1998. Effect of fasting and refeeding on growth and body composition of red porgy, Pagrus pagrus L. Aquacult Res 29:447–452.

Tian X, Qin JG. 2004. Effects of previous ration restriction on compensatory growth in barramundi Lates calcarifer. Aquaculture 235:273–283.

Wang Y, Cui Y, Yang Y, Cai F. 2000. Compensatory growth in hybrid tilapia, Oreochromis mossambicus×O. niloticus, reared in seawater. Aquaculture 189:101–108.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





