 |
 |
| Anim Biosci > Volume 34(1); 2021 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
ACKNOWLEDGMENTS
Figure 1
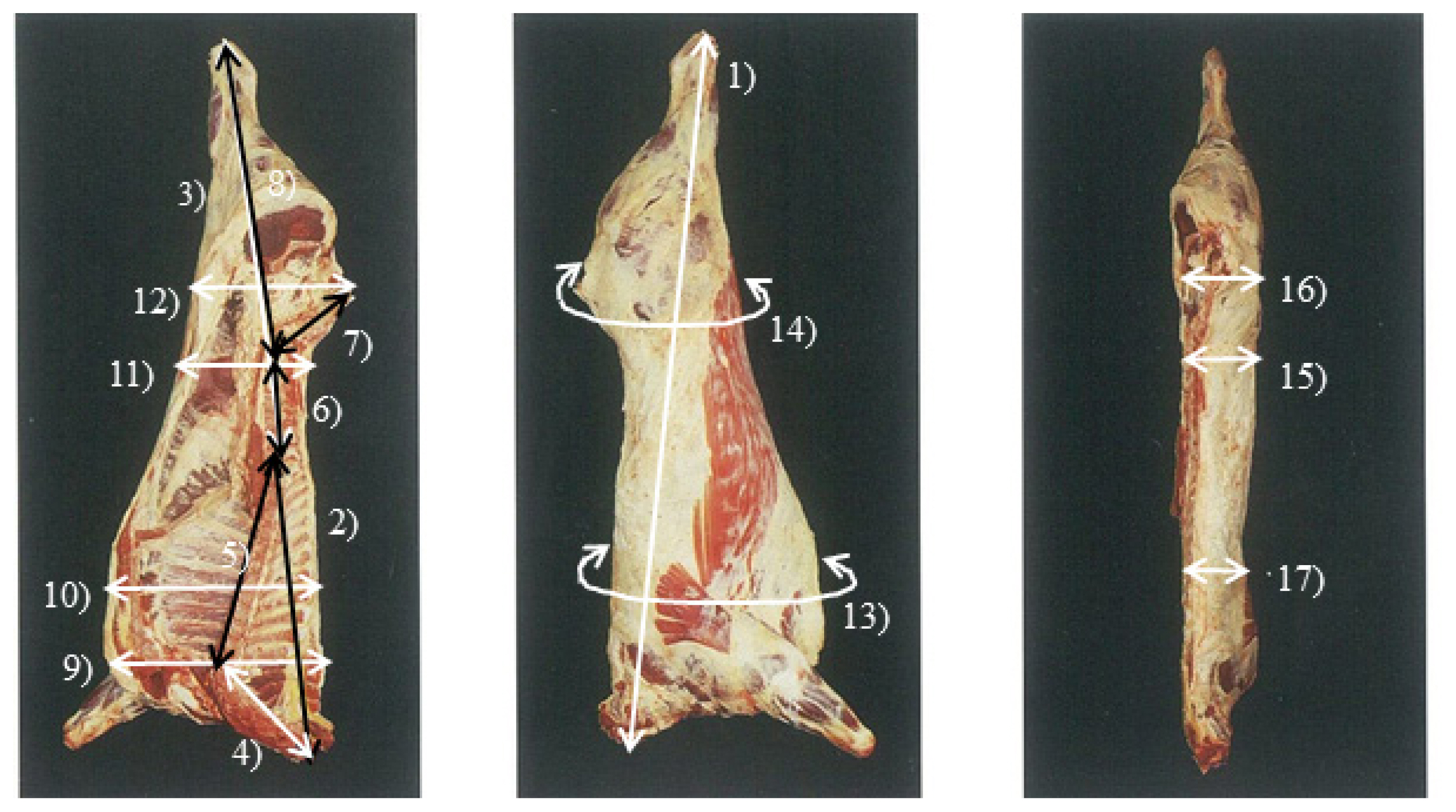
Table 1
Table 2
LW, live weight; CW, cold carcass weight; FT, carcass fat thickness; LMA, Longissimus dosi area; DP, dressing percentage (cold carcass weight×100/live weight); CL, carcass length; FL, forequarter length; HL, hindquarter length; CVL, cervical vertebrae length; TVL, thoracic vertebrae length; LVL, lumbar vertebrae length; SVL, sacral vertebrae length; LVHL, 6th lumbar vertebrae – heel length; CVB, 7th cervical vertebrae carcass breadth; TVG, 7th to 8th thoracic vertebrae girth; CG, coxae thick; LVT, coxae girth, 4th to 5th lumbar vertebrae thick; CT, coxae thick; TVT, 7th to 8th thoracic vertebrae thick.
1) Beef primal cuts [39].
Table 3
| Trait | Beef primal cuts1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Tenderloin | Loin | Strip loin | Chuck roll | Clod | Top round | Bottom round | Brisket | Shank | Ribs | |
| LW (kg) | 0.81*** | 0.76*** | 0.65*** | 0.46*** | 0.77*** | 0.81*** | 0.82*** | 0.75*** | 0.61*** | 0.65*** |
| CW (kg) | 0.78*** | 0.78*** | 0.72*** | 0.50*** | 0.72*** | 0.75*** | 0.76*** | 0.76*** | 0.67*** | 0.64*** |
| FT (mm) | 0.04 | 0.04 | 0.15 | −0.16 | −0.02 | −0.07 | −0.04 | 0.16 | 0.39** | 0.05 |
| LMA (cm2) | 0.54*** | 0.68*** | 0.73*** | 0.42*** | 0.50*** | 0.59*** | 0.59*** | 0.52*** | 0.24 | 0.55*** |
| DP (%) | 0.16 | 0.30* | 0.40** | 0.28* | 0.10 | 0.06 | 0.09 | 0.23 | 0.38** | 0.16 |
| Meat (g/kg) | 0.31* | 0.21 | 0.15 | 0.14 | 0.38** | 0.39** | 0.37** | 0.21 | −0.26* | 0.06 |
| Fat (g/kg) | −0.28* | −0.16 | −0.08 | −0.10 | −0.38** | −0.38** | −0.38** | −0.13 | 0.31* | −0.06 |
| Bone (g/kg) | 0.13 | 0.01 | −0.10 | −0.03 | 0.28* | 0.26* | 0.29* | −0.09 | −0.35** | 0.06 |
| CL (cm) | 0.69*** | 0.50*** | 0.41*** | 0.22 | 0.77*** | 0.70*** | 0.76*** | 0.55*** | 0.52*** | 0.43*** |
| FL (cm) | 0.56*** | 0.47*** | 0.33** | 0.39** | 0.57*** | 0.47*** | 0.56*** | 0.41** | 0.41** | 0.33* |
| HL (cm) | 0.50*** | 0.31* | 0.20 | 0.01 | 0.56*** | 0.50*** | 0.53*** | 0.38** | 0.35** | 0.30* |
| CVL (cm) | 0.23 | 0.24 | 0.13 | 0.20 | 0.17 | 0.24 | 0.23 | 0.15 | 0.23 | 0.14 |
| TVL (cm) | 0.42*** | 0.32** | 0.20 | 0.31* | 0.34** | 0.36** | 0.41** | 0.15 | 0.29* | 0.23 |
| LVL (cm) | 0.29* | 0.24 | 0.08 | 0.05 | 0.27* | 0.28* | 0.21 | 0.18 | 0.22 | 0.12 |
| SVL (cm) | 0.28* | 0.19 | 0.34** | 0.06 | 0.30* | 0.32* | 0.29* | 0.40* | −0.04 | 0.32* |
| LVHL (cm) | 0.57*** | 0.55*** | 0.41** | 0.29* | 0.59*** | 0.57*** | 0.58*** | 0.58*** | 0.33* | 0.48*** |
| CVB (cm) | 0.51*** | 0.45*** | 0.43*** | 0.35** | 0.52*** | 0.55*** | 0.58*** | 0.52*** | 0.44*** | 0.39** |
| TVB (cm) | 0.50*** | 0.47*** | 0.49*** | 0.41*** | 0.45*** | 0.64*** | 0.65*** | 0.38** | 0.41*** | 0.36** |
| LVB (cm) | 0.43*** | 0.29* | 0.46*** | 0.09 | 0.40** | 0.37** | 0.50*** | 0.35** | 0.33* | 0.21 |
| SVB (cm) | 0.35** | 0.32* | 0.32* | −0.16 | 0.35** | 0.26* | 0.29* | 0.38** | 0.22 | 0.32* |
| TVG (cm) | 0.59*** | 0.63*** | 0.54*** | 0.38** | 0.52*** | 0.61*** | 0.61*** | 0.61*** | 0.42*** | 0.62*** |
| CG (cm) | 0.44*** | 0.39** | 0.56*** | 0.52*** | 0.39** | 0.49*** | 0.53*** | 0.40** | 0.34** | 0.35** |
| LVT (cm) | 0.31* | 0.24 | 0.34** | −0.06 | 0.39** | 0.37** | 0.38** | 0.39** | 0.42*** | 0.11 |
| CT (cm) | 0.05 | 0.08 | 0.18 | −0.12 | 0.10 | 0.09 | 0.11 | 0.18 | 0.20 | −0.02 |
| TVT (cm) | 0.26* | 0.10 | 0.14 | −0.09 | 0.35** | 0.22 | 0.21* | 0.27* | 0.22 | 0.04 |
LW, live weight; CW, cold carcass weight; FT, carcass fat thickness; LMA, longissimus dosi area; DP, dressing percentage (cold carcass weight×100/live weight); CL, carcass length; FL, forequarter length; HL, hindquarter length; CVL, cervical vertebrae length; TVL, thoracic vertebrae length; LVL, lumbar vertebrae length; SVL, sacral vertebrae length; LVHL, 6th lumbar vertebrae – heel length; CVB, 7th cervical vertebrae carcass breadth; TVB, 5th to 6th thoracic vertebrae breadth; LVB, 4th to 5th lumbar vertebrae breadth; SVB, 5th Sacral vertebrae breadth; TVG, 7th to 8th thoracic vertebrae girth; CG, coxae thick; LVT, coxae girth, 4th to 5th lumbar vertebrae thick; CT, coxae thick; TVT, 7th to 8th thoracic vertebrae thick.
1) Beef primal cuts [39].






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





