 |
 |
| Anim Biosci > Volume 31(8); 2018 > Article |
|
Abstract
Objective
The effect of pre-converted nitrites from natural sources (spinach, lettuce, celery, and red beet) on color development in raw and cooked pork sausage was investigated in this study.
Methods
The pork sausage was manufactured with six treatments: NC (negative control, nitrite free), PC (positive control, 150 ppm sodium nitrite), FS (3.0% fermented spinach extracts), FL (3.0% fermented lettuce extracts), FC (3.0% fermented celery extracts), and FR (3.0% fermented red beet extracts).
Results
The pH value of the pre-converted nitrites groups was lower than those treated with 150 ppm sodium nitrite (p<0.05). The color values of raw and cooked pork sausage added with pre-converted nitrite showed slightly lower and/or similar lightness, lower redness, and higher yellowness values than PC. Color development (redness values) of cooked samples added with FS was higher than those of the NC and other treatments (FL, FC, and FR). Additionally, treatments with FS and FL were most effective for reducing thiobarbituric acid reactive substances and volatile basic nitrogen than the NC.
Conclusion
Effects of natural nitrites from fermented vegetables on shelf stability of raw and cooked pork sausages were investigated. Fermented spinach extract was much more useful for maintaining the color development, but also inhibiting lipid and protein oxidation of cooked pork sausage. Therefore, pre-converted nitrite from spinach as a natural nitrite could be used as another natural nitrite source for making processed meat products.
Recently, consumers have demanded healthier foods, preferably free of artificial additives, due to concerns about health risks, toxicity, and even carcinogenic effects of synthetic ingredients, such as sodium nitrite, butylated hydroxytoluene and butylated hydroxylanisole. For this reason, in the food industry, the attention of manufacturers has been shifted from synthetic to “healthy” (non-toxic) ingredients and the development of new preservative strategies [1,2].
Sodium nitrite, one of the main additives in meat product manufacturing, has four main functions: imparts a remarkable reddish pink color to the meat, imparts a characteristic flavor to meat products, reduces lipid oxidation, and controls the growth of several pathogenic and spoilage organisms [3]. The current approved levels of sodium nitrite in meat products are deemed safe, regardless of the pressure from consumer to further reduce or eliminate the use of sodium nitrite [4,5]. Also, the use of sodium nitrite in cooked meat products has been disputed due to the potential conversion of nitrite to nitrosamine, which is suspected of being a carcinogen [1]. Owing to this potential health risk, sodium nitrite alternatives from natural sources, which perceive to be healthier, are being developed for use in the meat-processing industry.
Alternative method to avoid the direct addition of sodium nitrite to meat products is by adding ingredients that have a high nitrate content and a starter culture to convert the nitrate to nitrite. According to previous researches, spinach, lettuce, celery, and red beet have excessive levels of nitrate of 2,400 ppm [6,5]. The pre-converted nitrite from celery is used at sufficient levels to maintain the pink color of meat products [7]. Previous study [6] noted that celery juice powder as a natural nitrite should be similar to sodium nitrite. However, as potential concerns have grown over the allergens of celery, meat-processing is actively seeking new sources of natural nitrite to increase color development of meat products.
Therefore, the purpose of this study was to estimate the effect of pre-converted nitrite from natural sources (red beet, lettuce, celery, and spinach) on physicochemical properties and color development in raw and cooked pork sausage and to compare the effects of synthetic nitrite with those of natural nitrite.
Commercial samples of spinach, lettuce, celery, and red beet were obtained from a local market. All raw samples were freeze-dried and ground into a powder form. Ten grams of each ground sample was mixed with ten-fold of distilled water for 30 min. Then, 0.025% active nitrate reductase culture containing Staphylococcus carnosus (S-B-61, Bactoferm, Chr. Hansen Inc., Gainesville, FL, USA) was added to the mixture, followed by incubation in a shaker incubator at 30°C for 24 h. Each mixture was filtered through Whatman No. 1 filter paper and evaporated with a rotary evaporator (EYELA N-1000, Rikakikai, Japan) at <50°C. After evaporation, nitrite contents of the fermented natural extracts were measured in order to confirm the conversion nitrate to nitrite from these ingredients before preparation of pork sausage for applying pre-converted nitrite (fermented spinach extract: nitrite content = 5,012.18 ppm, pH = 5.21, L*-value = 53.10, a*-value = −0.21, b*-value = 6.96; fermented lettuce extract: nitrite content = 2,200.93 ppm, pH = 4.77, L*-value = 22.40, a*-value = −2.03, b*-value = 20.31; fermented celery extract: nitrite content = 201.35 ppm, pH = 4.31, L*-value = 15.21, a*-value = 4.19, b*-value = 19.66; fermented red beet extract: nitrite content = 729.28 ppm, pH = 4.65, L*-value = 10.13, a*-value = 8.24, b*-value = 13.52). Concentrated products of the fermented natural extracts were then kept in the dark at 4°C until utilized within 24 h.
A total of three independent replicates of experiment were conducted. For each replicate, six batches (5 kg/batch) of pork sausages with different pre-converted nitrite from natural source were manufactured. Fresh pork ham (castrated boars, Landrace×Yorkshire×Duroc; approximately 110 kg, M. biceps femoris, M. semitendinosus, M. semimembranosus) and pork back fat (moisture 12.61%, fat 85.64%) were purchased from a local processor 48 h postmortem. Two pork hams (average weight, about 9 kg) from two carcasses were used in each replicate. Lean materials and pork fat were initially ground through an 8-mm plate. Ground tissues were placed in polyethylene bags, vacuum-sealed using a vacuum packaging system, and stored at 0°C until use. The experimental design and compositions of these pork sausage are shown in Table 1. The pork sausage without nitrite served as - controls (negative control, NC), and those with 150 ppm nitrite treatment served as +controls (positive control, PC). The different four groups of pork sausage were treated with pre-converted nitrite from natural source as follows: 3.0% fermented red beet extracts (FR), 3.0% fermented lettuce extracts (FL), 3.0% fermented celery extracts (FC), and 3.0% fermented spinach extracts (FS). Lean materials were homogenized and ground for 1 min and 30 s in a silent cutter (Nr-963009, Hermann Scharfen GmbH & Co., Postfach, Germany). Pork back fat, NaCl, and 0.15% sodium tripolyphosphate were added to the meat and mixed for 1 min and 30 s. Meat batters were homogenized for 3 min. A temperature probe (KM330, Kane-May, Harlow, UK) was used to monitor the temperature of the batter to maintain the temperature below 10°C throughout the batter preparation. After batter preparation, the meat batter was stuffed into collagen casings (#240, NIPPI Inc., Tokyo, Japan; approximate diameter, 25 mm) by IS-8 stuffer (Sirman, Marsango, Italy). Meat batters were heated at 80°C for 60 min in a chamber, and cooled at 21°C for 3 h. This procedure was performed in triplicates for each sausage sample [8].
The pH values of raw and cooked pork sausages were measured in a homogenate (UI-tra-Turrax T25, Janke & Kunkel, Staufen, Germany) prepared with 5 g of sample and 20 mL of distilled water using a pH meter (Model 340, Mettler-Toledo GmbH, Schwerzenbach, Switzerland). The pH meter calibrated with three different standard solutions (4.00, 7.02, and 10.05 pH buffers, VWR Scientific Products) at a temperature of 20°C±1°C. All determinations were performed in triplicate.
Color measurements were obtained with a colorimeter (Chroma meter CR-210, Minolta, Japan; illuminate C, calibrated with a white standard plate L* = 97.83, a* = −0.43, and b* = +1.98) using an 8-mm-diameter measuring area and a 50-mm-diameter illumination area. Color was expressed with L* (100 = white, 0 = black), a* (positive = redness, negative = greenness), and b* (positive = yellowness, negative = blueness) values. Color readings were measured on ten randomly chosen spots on the model systems and were utilized as an estimate of discoloration. The total color differences between the NC (no nitrite) and treatments were calculated by: ΔE = [(L*–LNC*)2 +(a*–aNC*)2+(b*–bNC*)2]½. Additionally, the hue (H°) and chroma (C*: saturation) values were evaluated using the formula, Tan−1(b*/a*) and (a2+b2)1/2 respectively [9].
Lipid oxidation was evaluated in triplicate using the thiobarbituric acid reactive substances (TBARS) method of Tarladgis et al [10] with minor modifications and was calculated as milligrams of malondialdehyde (MD) per kilogram of sausage. A 10 g sample was blended using homogenizer (AM-7, Nihonseiki, Kaisha Ltd., Tokyo, Japan) with 50 mL distilled water for 2 min and then transferred to a distillation flask. The cup used for blending was washed with an additional 47.5 mL of distilled water and added to the same distillation flask with 2.5 mL of 4 N HCl and a few drops of antifoaming agent (KMK-73, Shin-Etsu Silicone Co., Ltd., Seoul, Korea). The mixture was distilled, and 50 mL of the distillate was collected. Five milliliters of 0.02 M thiobarbituric acid (TBA) in 90% acetic acid (TBA reagent) was added to each test tubes containing 5 mL of the distillate and mixed well. The tubes were capped and heated in a boiling water bath for 30 min to develop the chromogen and cooled to room temperature. Absorbance was measured at 538 nm against a blank prepared with 5 mL distilled water and 5 mL TBA reagent using a UV/VIS spectrophotometer (Optizen 2120 UV plus, Mecasys Co. Ltd., Seoul, Korea).
Volatile basic nitrogen (VBN) (mg %) test was conducted to determine the extent of protein deterioration during refrigerated storage. VBN was measured by the modified micro diffusion assay according to the method of Pearson [11].
Where, a = titer for sample, b = titer for blank, f = factor of reagent, N = normality, S = sample weight (g).
For all microbial counts, 25 g of samples were weighed and transferred into a sterile stomacher bag containing 225 mL of 0.1% peptone water followed by pummeling samples in a stomacher (Masticater-Paddle-Blender, IUL Instrument, Barcelona, Spain) for 2 min. From the prepared dilutions, total viable count (TVC), total coliform bacteria count (TCC) and Escherichia coli (E.coli) were carried out. Plate Count Agar (PCA; Difco, Sparks, MD, USA) was used for TVC with incubation periods of 37°C for 48 h. E. coli/coliform count plate petrifilm (3M Healthcare, MN, USA) was used for E. coli and TCC, respectively, with an incubation period of 35°C for 24 h under the same aerobic conditions.
Residual nitrite content was determined according to the AOAC [12] and is expressed as ppm of model systems. All residual nitrite assays were done in duplicated and all treatments within a block were analyzed at the same time to minimize variation in the analysis due to time. The residual nitrite content was calculated by a standard curve using nitrite solution (KFDA [13]).
The effect of natural pre-converted nitrite sources (spinach, lettuce, celery, and red beet) on pH, color, TBARS, VBN, TVC, E. coli, TCC, and residual nitrite contents was examined using a one way analysis of variance, where the measured variables were set as dependent variables, different fermented extracts as fixed effect, and replicate as random effect. Differences among the means were compared by using Duncan’s multiple range test. A significance level of p<0.05 was used for all evaluations. The data was analyzed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). The values were given in terms of mean values and standard error in tables and figures. The entire trial was replicated thrice. Least squares mean for all traits were separated (F test, p<0.05) by using least significant differences.
Addition of natural pre-converted nitrite extracts in raw and cooked samples did significantly affect the pH values (Tables 2, 3). In these studies, the pH values of both raw and cooked pork sausage treated with PC (150 ppm nitrite) were higher than those of the NC (no nitrite) and other treatments (p< 0.05). This trend may be due to natural pre-converted nitrite extracts that have low pH values (fermented spinach extract, pH = 5.21; fermented lettuce extract, pH = 4.77; fermented celery extract, pH = 4.31; fermented red beet extract, pH = 4.65). Results are in accordance with those of previous study [6], which noted that bologna with celery juice powder has lower pH values than the control samples and it could mainly cause the increase in Log CFU/g of lactic acid bacteria. Sindelar et al [14] observed that the pH values were significantly influenced by pre-converted nitrite from celery when ham incubated different levels of celery powder. The decreasing trend in pH values supports the view that the formation of organic acid by bacterial metabolism caused decrease in pH, which may inhibit the coliform growth [15]. Pexara et al [16] reported that a decrease in the pH of meat depends on the availability of fermentable carbohydrates.
When compared to pH differences between raw and cooked sausages, the pH values in cooked samples usually increase owing to the imidazolium that is unfolded and exposed, which acts as a basic activity due to the histidine [17].
The color values of raw and cooked pork sausage have been influenced by the pre-converted nitrite from natural sources because all treatments show slightly lower and/or similar lightness, lower redness, and higher yellowness values than PC (Tables 2, 3). This finding agrees with the results of Djeri and Williams [6]. They reported that the bologna sausage added with celery juice powder had significantly darker color compared to control containing sodium nitrite and all treated samples. Conversely, Sindelar et al [14] reported no difference in lightness of ham slices between the control treated with sodium nitrite and samples incubated with celery juice powders.
As expected, PC (150 ppm nitrite) of cooked pork sausage had a more intense red color than NC and other samples. The formulations with FL (fermented lettuce extract) indicated lower redness in raw pork batters compared to that of the NC, PC, and other treatments. After cooking, pork sausage added with FS results in higher redness than the samples containing with natural pre-converted nitrite sources from spinach, celery, and red beet. Terns et al [18] reported that cured pigment degradation was related to decrease in redness during storage. They also expected higher redness with more rapid formation of cured pigment aided by addition of natural cure accelerator such as cherry powder. Results are in accordance with those of Sindelar et al [14], who reported that natural cure accelerators are not only rich in ascorbic acid (vitamin C) which functions as a strong nitrite-reducing agent but also characterized by low pH which promotes nitric oxide production that depletes residual nitrite. Previous research proposed that the redness may positively correspond to reflectance ratios, whereas an increase in lightness may negatively affect cured pigment measurements, as determined by the reflectance ratio [14].
The hue angle index (H°) and color difference (ΔE*) of the raw and cooked pork samples showed some significant differences in relation to natural pre-converted nitrite sources. In the hue angle index, higher values indicate browner hues, and color differences among higher values show greater total color differences [19]. In this study, the both raw and cooked pork sausage treated with the pre-converted nitrite had higher hue angle indices and color differences than those for the NC. It could be reported that fermented natural extracts contribute to the typical brownness color (fermented spinach extract, b*-value = 7.07; fermented lettuce extract, b*-value = 20.31; fermented celery extract, b*-value = 19.66; fermented red beet extract, b*-value = 13.52), resulting in a discoloration of the raw and cooked pork meat.
The VBN of the cooked pork sausage formulated with natural pre-converted nitrite sources are shown in Table 4. According to Kohsaka [20], the VBN value could be utilized as an important indicator of deterioration of meat product freshness during storage periods, being affected by amino acid decarboxylase as well as enzymes and microorganisms [21]. The lowest VBN values were observed in the PC of cooked samples, and the VBN of the pre-converted nitrite group, except for FC, were lower than those of the NC (p<0.05). Weiss et al [3] assumed that primary functions of nitrite are to prevent the growth of foodborne pathogens and to the metabolism of proteolytic enzymes in meat products, thus decreasing their VBN values. Jay [22] reported that the formation of volatile compounds is strongly related to the growth of microorganisms that cause an increase in the VBN value of meat products; therefore, inhibition of microbial growth since the nitrite content could explain the lower formation of volatile compounds.
Lipid oxidation not only accelerate rancidity by heat treatment but also changes their nutritive value, color, and flavor [22,23]. The effects of natural pre-converted nitrite sources on the TBARS of the cooked pork sausage are summarized in Table 4. As expected, the PC appeared to effectively retard MA concentrations of cooked pork samples whereas lipid oxidation in the NC was more intense compared to other treatments. Sindelar et al [14] reported no significant difference between treatments containing sodium nitrite or celery juice powders. In these studies, the pre-converted nitrite from spinach (FS) and lettuce (FL) have lower TBARS values of pork sausages. This could be due to residual nitrite contents of samples with FS (491.68 ppm) or FL (220.09 ppm) which may affect the oxidative stability of cooked pork samples. In general, nitrite has been shown to be an effective antioxidant [24]. Tarladgis et al [10] reported that TBARS of 0.5 to 1.0 mg MD/kg is considered the threshold for oxidized odor and 1.0–2.0 mg MD/kg for oxidized flavor. The TBARS values of all cooked samples maintained TBARS values below 0.5 mg/kg.
TVC and numbers of E. coli and TCC in the cooked pork samples treated with natural pre-converted nitrite sources are shown in Table 5. The TVC of PC, NC, and all treated samples were 1.42 to 2.70 log CFU/g and a small but significant (p<0.05) inhibitory effect of the pre-converted nitrite group on TVC when applied to the cooked pork sausage. E. coli and TCC of cooked sausage were not detected in all treatments. Djeri and Williams [6] described that meat products with added both celery juice and cherry powder indicated lower anaerobic counts on all storage days, due to the sodium nitrite of these powders. Jackson et al [25] reported that frankfurters cured with a natural nitrate source (celery powder) and a starter culture was significantly reduced C. perfringens growth during the entire storage period (8 days). Also, they reported that the antimicrobial action of pre-converted nitrite from celery was very effective. In these results, all treatments are expected to show lower levels of the initial TVC.
The effects of the natural pre-converted nitrite sources on the residual nitrite content of the cooked pork sausage are shown in Figure 1. The highest residual nitrite content was found in the cooked sample added with 150 ppm sodium nitrite (PC), whereas the lowest was observed in treatments without nitrite (NC). The residual nitrite level of pre-converted nitrite group decreased in the following order: FS (Fermented spinach)>FL (Fermented lettuce) >FC (Fermented celery)>FR (Fermented red beet). For this reason, the residual nitrite contents probably are related to the conversion rate of nitrate into nitrite during the fermentation, which could affect final nitrite contents in each vegetable extracts. These results are in agreement with the findings by Sindelar et al [26], who reported that the residual nitrite content of sausages containing concentrated celery juice powder yielded a greater amount of nitrite converted from nitrate during incubation. Further, some researchers observed decreasing residual nitrite levels with food processing such as curing, heating, smoking, and packaging, and the nitrite concentration was affected by both storage time and temperature [27,28].
This study reveals that pre-converted nitrites from spinach, lettuce, celery, and red beet have significant potential for use in raw and cooked pork sausage as a natural nitrite. Among the pre-converted nitrites, FS showed the most potential as alternatives to conventional sodium nitrite, not only maintaining the color development, but also inhibiting lipid and protein oxidation of cooked pork sausage.
ACKNOWLEDGMENTS
This research was supported by Main Research Program (E0156422-04) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science, ICT & Future Planning (Republic of Korea). This research was also partially supported Agri-Bio Industry Technology Development Program (317001-3) by the Ministry of Agriculture, Food and Rural Affairs (Republic of Korea).
Figure 1
Effects on residual nitrite contents of cooked meat model systems formulations with fermented natural extracts. 1) NC, negative control (nitrite free); PC, positive control (150 ppm nitrite); FS, fermented spinach extract; FL, fermented lettuce extract; FC, fermented celery extract; FR, fermented beet extract. A–E Means with different letters are significantly different (p<0.05). * Standard error.
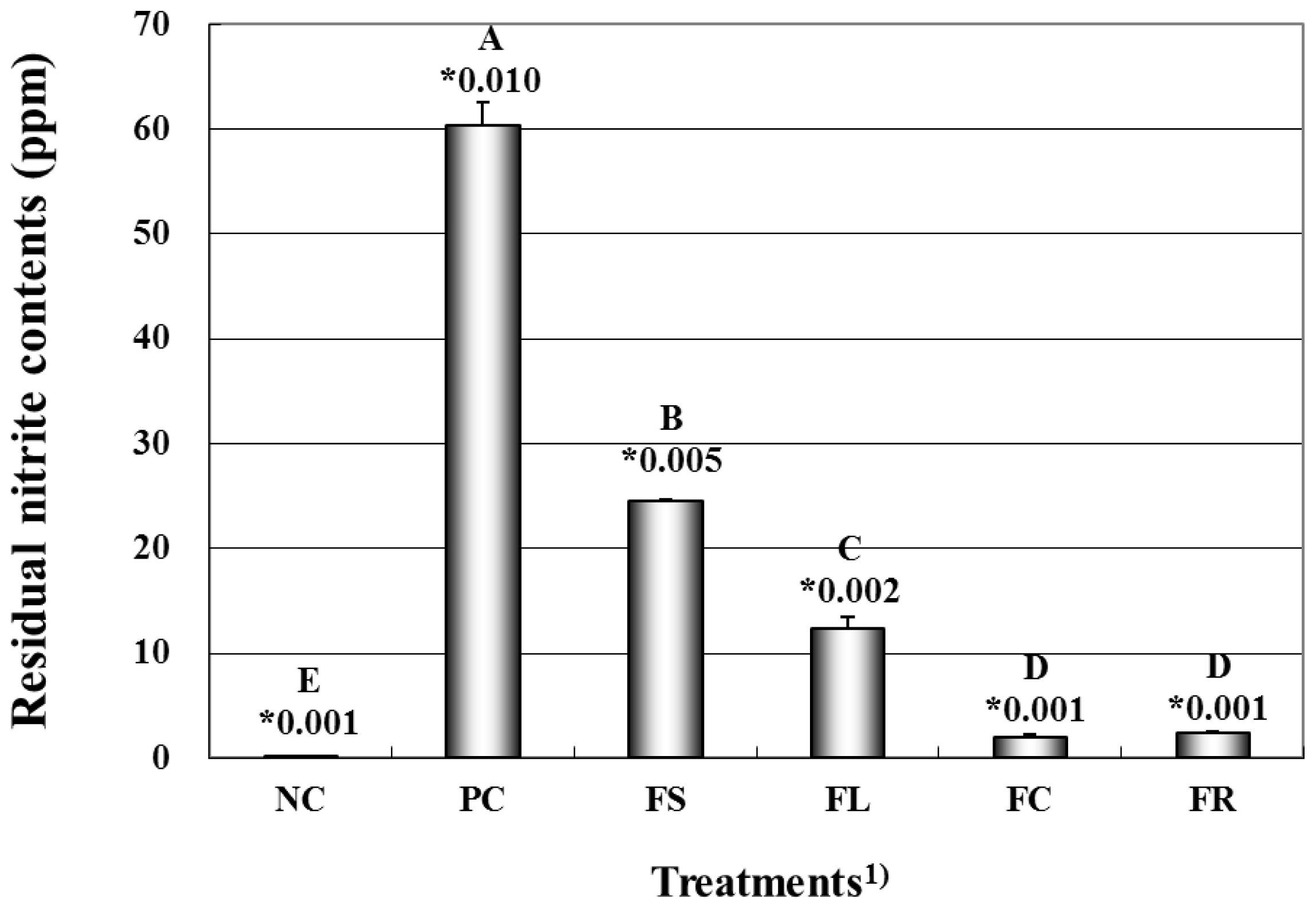
Table 1
Pork sausage formulations using pre-converted nitrite from natural source (units: g/100 g)
| Ingredients | Treatments1) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| NC | PC | FS | FL | FC | FR | |
| Pork ham | 50 | 50 | 50 | 50 | 50 | 50 |
| Back fat | 25 | 25 | 25 | 25 | 25 | 25 |
| Ice | 25 | 25 | 25 | 25 | 25 | 25 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Sodium chloride | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Sodium tripolyphosphate | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Sodium nitrite | - | 0.015 | - | - | - | - |
| Fermented spinach extract | - | - | 3.0 | - | - | - |
| Fermented lettuce extract | - | - | - | 3.0 | - | - |
| Fermented celery extract | - | - | - | - | 3.0 | - |
| Fermented red beet extract | - | - | - | - | - | 3.0 |
Table 2
Effects of using pre-converted nitrite from natural source on pH and color of in raw pork batter
| Treatments1) | pH | Color | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| L*-value | a*-value | b*-value | H°2) | ΔE*3) | ||
| NC | 6.41±0.00c | 61.1±0.13b | 11.8±0.11a | 12.3±0.13d | 46.1±0.27f | - |
| PC | 6.48±0.02a | 62.1±0.04a | 10.1±0.06b | 11.6±0.08e | 49.2±0.10e | 2.2±0.14e |
| FS | 6.46±0.01b | 62.0±0.18a | 8.9±0.07c | 12.8±0.07c | 55.2±0.30d | 3.2±0.12d |
| FL | 6.45±0.00b | 58.3±0.04c | 3.5±0.01e | 13.7±0.05b | 75.9±0.14a | 9.0±0.11a |
| FC | 6.02±0.00e | 61.3±0.07b | 9.2±0.02c | 15.1±0.03a | 58.6±0.16c | 4.0±0.05c |
| FR | 6.34±0.00d | 61.5±0.24ab | 7.3±0.08d | 14.7±0.07a | 63.6±0.21b | 5.3±0.13b |
| SEM | 0.039 | 0.148 | 0.278 | 0.137 | 1.281 | 0.340 |
| p value | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
Table 3
Effects of using pre-converted nitrite from natural source on pH and color of in cooked pork sausage
| Treatments1) | pH | Color | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| L*-value | a*-value | b*-value | H°2) | ΔE*3) | ||
| NC | 6.50±0.00b | 66.3±0.02a | 3.2±0.01d | 11.2±0.03d | 74.0±0.11c | - |
| PC | 6.55±0.01a | 66.1±0.12a | 9.8±0.06a | 9.5±0.01e | 44.0±0.23e | 6.8±0.10a |
| FS | 6.53±0.00ab | 66.0±0.01a | 4.8±0.03b | 11.8±0.03c | 68.0±0.19d | 2.0±0.12d |
| FL | 6.51±0.00b | 62.8±0.04b | 0.3±0.00f | 13.5±0.10ab | 89.0±0.04a | 5.2±0.09b |
| FC | 6.40±0.01d | 66.5±0.16a | 3.8±0.01c | 13.1±0.21b | 73.7±0.25c | 2.2±0.21d |
| FR | 6.45±0.02c | 66.7±0.10a | 3.4±0.03d | 13.7±0.06a | 76.1±0.22b | 2.6±0.11c |
| SEM | 0.013 | 0.146 | 0.303 | 0.163 | 1.766 | 0.281 |
| p value | 0.001 | 0.012 | 0.001 | 0.001 | 0.001 | 0.001 |
Table 4
Effects of using pre-converted nitrite from natural source on VBN and TBARS values in cooked pork sausage
| Treatments1) | VBN (mg %) | TBARS (mg MA/kg) |
|---|---|---|
| NC | 3.79±0.30b | 0.39±0.00a |
| PC | 1.76±0.11d | 0.06±0.00c |
| FS | 2.33±0.11cd | 0.16±0.01b |
| FL | 2.13±0.11cd | 0.22±0.03b |
| FC | 5.26±0.20a | 0.36±0.01a |
| FR | 2.30±0.23cd | 0.38±0.00a |
| SEM | 0.218 | 0.326 |
| p value | 0.001 | 0.001 |
VBN, volatile basic nitrogen; TBARS, thiobarbituric acid reactive substances; SEM, standard error of means.
Table 5
Effects of using pre-converted nitrite from natural source on total viable count, Escherichia coli, and total coliform bacteria in cooked pork sausage
| Treatments1) | Total viable count (log CFU/g) | Escherichia coli (log CFU/g) | Total coliform bacteria (log CFU/g) |
|---|---|---|---|
| NC | 2.10±0.11a | ND | ND |
| PC | 1.61±0.19ab | ND | ND |
| FS | 1.42±0.03b | ND | ND |
| FL | 1.68±0.06ab | ND | ND |
| FC | 1.64±0.12b | ND | ND |
| FR | 1.78±0.08b | ND | ND |
| SEM | 0.070 | - | - |
| p value | 0.006 | - | - |
REFERENCES
1. Bedale W, Sindelar JJ, Milkowski AL. Dietary nitrate and nitrite: benefits, risks, and evolving perceptions. Meat Sci 2016; 120:85–92.


2. Hwang KE, Kim HW, Choi YS, et al. Evaluation of the antioxidant effect of ganghwayakssuk (Artemisia princeps Pamp.) extract alone and in combination with ascorbic acid in raw chicken patties. Poult Sci 2013; 92:3244–50.



3. Weiss J, Gibis M, Schuh V, et al. Advances in ingredient and processing systems for meat and meat products. Meat Sci 2010; 86:196–213.


4. Kang JO, Lee GH. Effects of pigment of red beet and chitosan on reduced nitrite sausages. Korean J Food Sci An 2003; 23:215–20.
5. Walker R. Nitrates, nitrites and N-nitrosocompounds: a review of the occurrence in food and diet and the toxicological implications. Food Addit Contam 1990; 7:717–68.


6. Djeri N, Williams SK. Celery juice powder used as nitrite substitute in sliced vacuum-packaged turkey bologna stored at 4C for 10 weeks under retail display light. J Food Qual 2014; 37:361–70.

7. Krause BL, Sebranek JG, Rust RE, et al. Incubation of curing brines for the production of ready-to-eat, uncured, no-nitrite-or-nitrate-added, ground, cooked and sliced ham. Meat Sci 2011; 89:507–13.


8. Choi YS, Choi JH, Han DJ, et al. Characteristics of low-fat meat emulsion systems with pork fat replaced by vegetable oils and rice bran fiber. Meat Sci 2009; 82:266–71.


9. Grigelmo-Miguel N, Abadías-Serós MI, Martín-Belloso O. Characterisation of low-fat high-dietary fibre frankfurters. Meat Sci 1999; 52:247–56.


10. Tarladgis BG, Watts BM, Younathan MT, et al. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J Am Oil Chem Soc 1960; 37:44–8.

11. Pearson D. Assessment of meat freshness in quality control employing chemical techniques: a review. J Sci Food Agric 1968; 19:357–63.

12. AOAC. Nitrites in cured meat Official Method 973. 31. Official methods of analysis. 15th edAssociation of Official Analytical Chemists. Washington DC, USA: AOAC International; 1990.
13. KFDA. Korean Food Standards, Analytical methods of residual nirite in foods. Korea Food and Drug Administration (KFDA); 2016.
14. Sindelar JJ, Cordray JC, Sebranek JG, et al. Effects of varying levels of vegetable juice powder and incubation time on color, residual nitrate and nitrite, pigment, ph, and trained sensory attributes of ready-to-eat uncured ham. J Food Sci 2007; 72:S388–95.


15. Kim TK, Kim YB, Jeon KH, et al. Effect of fermented spinach as sources of pre-converted nitrite on color development of cured pork loin. Korean J Food Sci Anim Resour 2017; 37:105–13.




16. Pexara ES, Metaxopoulos J, Drosinos EH. Evaluation of shelf life of cured, cooked, sliced turkey fillets and cooked pork sausages—‘piroski’—stored under vacuum and modified atmospheres at +4 and +10°C. Meat Sci 2002; 62:33–43.


17. Choi YS, Choi JH, Han DJ, et al. Effects of rice bran fiber on quality of low-fat tteokgalbi. Food Sci Biotechnol 2008; 17:959–64.
18. Terns MJ, Milkowski AL, Rankin SA, et al. Determining the impact of varying levels of cherry powder and starter culture on quality and sensory attributes of indirectly cured, emulsified cooked sausages. Meat Sci 2011; 88:311–8.


19. Hunt MC, Sørheim O, Slinde E. Color and heat denaturation of myoglobin forms in ground beef. J Food Sci 1999; 64:847–51.

20. Kohsaka K. Freshness preservation of food and measurement. Food Ind 1975; 18:105–111.
21. Kim HW, Choi YS, Choi JH, et al. Effects of rice bran fiber on changes in the quality characteristics of raw ground pork during chilled storage. Korean J Food Sci Anim Resour 2011; 31:339–48.


22. Jay JM. Indicators of food microbial quality and safetyJay JM, Loessner MJ, Golden DA, editorsModern food microbiology. Boston, MA, USA: Springer; 1995. p. 387–407.
23. Choi YS, Choi JH, Kim HY, et al. Effect of lotus (Nelumbo nucifera) leaf powder on the quality characteristics of chicken patties in refrigerated storage. Korean J Food Sci Anim Resour 2011; 31:9–18.


24. Shahidi F, Hong C. Evaluation of malonaldehyde as a marker of oxidative rancidity in meat products. J Food Biochem 1991; 15:97–105.

25. Jackson AL, Kulchaiyawat C, Sullivan GA, et al. Use of natural ingredients to control growth of Clostridium perfringens in naturally cured frankfurters and hams. J Food Prot 2011; 74:417–24.


26. Sindelar JJ, Cordray JC, Olson DG, et al. Investigating quality attributes and consumer acceptance of uncured, No-nitrate/nitrite-added commercial hams, bacons, and frankfurters. J Food Sci 2007; 72:S551–9.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





