 |
 |
| Anim Biosci > Volume 31(1); 2018 > Article |
|
Abstract
Objective
The objective of this research was to evaluate the physico-chemical, microbiological and sensorial qualities of restructured steaks processed from beef trimmings (grade I and II) and frozen beef (fresh beef as control and frozen beef).
Methods
Beef trimmings from commercial butcher were collected, designated into 4 treatments differing in beef trimmings grade and freezing, processed into restructured steaks with 1% microbial transglutaminase and then analyzed for product quality.
Results
The results showed that all meat from different groups could be tightly bound together via cross-linking of myosin heavy chain and actin as observed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Microbial counts of psychrotrophic and mesophilic bacteria were not affected by treatments (p>0.05), and no detectable of thermophilic bacteria were found. Regarding effect of beef trimmings grade, steaks made from beef trimmings grade II (16.03% fat) showed some superior sensorial qualities including higher tenderness score (p<0.05) and tendency for higher scores of juiciness and overall acceptability (p<0.07) than those made from beef trimmings grade I (2.15% fat). Moreover, a hardness value from texture profile analysis was lower in steaks processed from beef trimmings grade II than those made from grade I (p< 0.05). Although some inferior qualities in terms of cooking loss and discoloration after cooking were higher in steaks made from beef trimmings grade II than those made from beef trimmings grade I (p<0.05), these differences did not affect the sensory evaluation. Frozen beef improved the soft texture and resulted in effective meat binding as considered by higher cohesiveness and springiness of the raw restructured product as compared to fresh beef (p<0.05).
Conclusion
The results indicated the most suitable raw beef for producing restructured steaks without detrimental effect on product quality was beef trimmings grade II containing up to 17% fat which positively affected the sensory quality and that frozen beef trimmings increased tenderness and meat binding of restructured beef steaks.
The demand for beef consumption was growing worldwide at an estimated 0.37% per year during 2010 thorugh 2014 [1]. Additionally, the increasing costs of beef production have prompted the industry to develop strategies to utilize low-value meat cuts and beef trimmings to generate additional revenue. Restructured meat, prepared from small cuts of meat in an effort to increase the yield of marketable products, offers many advantages for both consumer and the meat industry. Since there is no added sodium chloride or phosphates and uses commercial microbial transglutaminase (MTGase) as a binding agent, restructured meat products are considered ŌĆśhealthyŌĆÖ as reported by Kuraishi et al [2].
MTGase is an enzyme promoting protein binding in muscle foods through covalent cross-linking between glutamine and lysine residues, resulting in the formation of high molecular weight polymers [3]. MTGase is active at the pH range 5 to 8 and temperature range 2┬░C to 6┬░C. This enzyme is widely utilized for the restructuring of meat by binding together small pieces of meat and the quality of product can be varied depended on intrinsic properties of meat like muscle fiber type [4] meat particle size [5], alignment of muscle fiber [6], and post-mortem aging time [7] as well as extrinsic factors of non-meat ingredients and processing conditions [8]. However, the quality of products containing MTGase as impacted by beef trimmings grade and frozen beef has not been evaluated.
Generally, the quality of beef trimmings is classified based on the maximum amount of fat that it is allowed to contain, which differs between countries. According to Heinz and Hautzinger [9], there are three grades of manufacturing meat from cattle including grade B1 (lean beef without visible fat), grade B2 (less than 10% fat) and grade B3 (less than 20% fat), which beef trimmings with higher fat content are classified as lower-grade meat due to larger amounts of visible connective tissue. In Thailand, Sethakul and Sivapirunthep [10] categorized beef trimmings into 5 groups including RI, RII, RIII, RIV and RV that contained 5%, 10%, 15%, 30%, and 35% fat content, respectively. Several studies have shown that increased fat levels increase consumer acceptance of fresh beef steaks [11,12]. However, for restructured meat in which fat can interfere with meat binding, it is essential to evaluate which grades of beef trimmings can be formulated with enhanced product quality or at least no detrimental impact on product quality.
Frozen meat has an advantage over fresh meat with increased storage time and a greater flexibility in inventory for retailers [13], but it reduces meat quality. The cell membranes of meat are damaged upon freezing which results in a lower water-holding capacity and a higher cooking loss [13] and the consequently production of less juicy meat [14]. Therefore, the aim of the present experiment was to evaluate the quality of restructured beef steak in terms of physico-chemical, microbiological and sensorial properties as influenced by the use of beef trimmings grades and frozen beef.
Fresh beef trimmings were obtained from sirloin and round portions with two commercial-quality grades; beef trimmings grade I (lean with fat content up to 10%) and grade II (lean with fat content up to 20%) (Figure 1). These samples were collected from a commercial butcher, Pon Yang Kham Livestock Breeding Cooperatives NSC Ltd., Pathumthani, Thailand. Beef trimmings were transported on ice to the meat laboratory of the Department of Animal Production Technology and Fisheries, King MongkutŌĆÖs Institute of Technology Ladkrabang (KMITL) within 4 h for further processing. Then, beef trimmings grades I and II were analyzed for meat pH and proximate composition.
The ultimate pH of meat was directly measured at three different locations using portable pH meter (Mettler Toledo SevenGo SG2, Mettler Toledo, Schwerzenbach, Switzerland).
Moisture, protein, fat and ash contents of beef trimmings were analyzed according to the method of AOAC [15]. The values were expressed as % (wet weight basis). Triplicate determinations were done for each meat.
To study effects of beef trimmings grade (grade I and II) and frozen beef (fresh as a control and frozen beef) on the resulting quality of restructured beef, a total of 4 different treatments were performed. Each type of beef trimmings was divided into two groups. The first group of the sample was chilled at 4┬░C for 24 h and assigned to fresh samples. The residual sample was frozen at ŌłÆ20┬░C for 1 month and then thawed with running tap water until attained 4┬░C of core temperature (CT), which was simulated as freeze-thawed beef or frozen samples. Thereafter, 4 different restructured beef steaks were formulated as shown in Table 1. Cold-set restructured beef steaks were made according to the procedure of Farouk et al [6] with some modification. Briefly, beef trimmings were striped into about 20├Ś60├Ś10 mm and a 2-kg restructured beef was manufactured. The samples (2-kg) were thoroughly mixed with 3% (w/w) of chilled water and 1% (w/w) of MTGase (ACTIVA TG-B Powder Sprinkle QS-Type, Ajinomoto Co., Ltd., Bangkok, Thailand) using a bowl-lift stand mixer (KitchenAid, Professional 600, St. Joseph, MI, USA) for 4 min at a speed setting of 2. The resulting mixture was stuffed into a stainless steel ham mould/press. The stuffed moulds were held for 6┬░C to 8┬░C for 4 h to allow the binder to bind the restructured meat pieces. The reformed meats were removed from mould and then packed in vacuum bags and stored at ŌłÆ20┬░C until analysis. A frozen sample was semi-thawed to the temperature of ŌłÆ2┬░C to 0┬░C and then sliced into a 20-mm thick steaks for further analysis. Three replications of each treatment were made to determine differences among batches (n = 3).
Protein pattern of raw beef and resulting raw restructured beef was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 4% stacking and 10% running gels according to the method of Laemmli [16]. To solubilize the sample, 27 mL of 5% (w/v) SDS were added into 3 g of sample. Mixtures were homogenized for 1 min at a speed of 12,000 rpm and incubated at 85┬░C for 1 h to dissolve total proteins followed by centrifuging the samples at 3,500 g for 20 min to remove undissolved debris. Dissolved protein was frozen and stored at ŌłÆ20┬░C until analysis. Proteins (15 ╬╝g) determined by the Lowry method [17] were loaded onto the gel and subjected to electrophoresis at a constant current of 20 mA per gel using a vertical gel electrophoresis, miniPAGE chamber AE-6530 (ATTO Corporation, Tokyo, Japan). After separation, the protein bands were stained overnight using 0.125% (w/v) Coomassie Brilliant Blue R-250 in 45% (v/v) ethanol and 10% (v/v) acetic acid. Destaining was performed using 30% (v/v) methanol and 10% (v/v) acetic acid.
Eight pieces of raw steaks per treatment were weighed and grilled in a pan until the CT reached 71┬░C, as monitored by probes of Type-K thermocouple from a digital thermometer (52 Series II, Fluke Corp., Everett, WA, USA). Cooked steaks were cooled for 30 min at 25┬░C and weighed. Cooking loss was calculated from the differences in the weight of raw and cooked steaks and expressed as the percentage of initial weight. Cooked steaks were used to further analysis of color, instrumental texture, microbiological analysis and sensory evaluation.
The color of four samples from raw and cooked restructured steaks was measured in the L* a* b* mode of Commission Internationale de lŌĆÖEclairage (CIE) by a color measurement spectrophotometer (MiniScan EZ, HunterLab, Reston, VA, USA). Three locations per sample were carried out and the resulting average was used in data analysis, where the data was expressed as CIE L* (lightness), a* (redness), and b* (yellowness).
The WarnerŌĆōBratzler shear force (WBSF) was determined in raw and cooked restructured steaks. Ten rectangular cube samples for each steak (10 mm├Ś10 mm├Ś25 mm) were taken. Each sample was sheared with a WBSF device attached to an Instron universal testing machine (3344, Instron Engineering Corp., Canton, MA, USA) with a 50-kg load cell using a crosshead speed of 60 mm/min. The maximum force (N) was recorded.
The raw and cooked steaks were subjected to texture profile analysis (TPA) [18] using an Instron universal testing machine model 3344 with a compression plate surface. Steaks at 25┬░C were cut into 10 cube samples (20├Ś20├Ś20 mm) and placed on the instrumentŌĆÖs base. TPA textural parameters were measured at room temperature with the following testing conditions: crosshead speed was 60 mm/min and compressed twice to 40% of their original height. The Bluehill 2 software (Instron Engineering Corp., USA) was used to collect and process the data. TPA analyses were defined and calculated as previously described by Bourne [18]. Hardness (N), cohesiveness (ratio), gumminess (N), springiness (ratio), and chewiness (N) were calculated from the force-time curves generated for each sample.
Aerobic bacteria counts of raw and cooked steaks from 4 treatments were carried out. Each sample (25 g) was transferred into 225 mL of 0.85% NaCl and homogenized for 2 min with the Stomacher BagMixers 400 VW (Interscience Co., Saint-Nom-la-Bret├©che, France). Appropriate ten-fold dilutions of the samples were prepared in 0.85% NaCl and dropped on growth media in duplicate to estimate microbial counts. Total psychrotrophic, mesophilic and thermophilic aerobic populations were estimated on plate count agar (PCA) incubated at 7┬░C for 10 days, 37┬░C for 24 to 48 h and 55┬░C for 3 days, respectively. The number of colonies was counted and expressed as logarithms of colony forming units per gram (Log CFU/g) [19].
Sensory attributes of cooked steaks in regard to color, appearance, flavor, tenderness, juiciness and overall acceptability of the sample, were evaluated by 30 semi-trained panelists from undergraduate and graduate students of Department of Animal Production Technology and Fishery, KMITL using a seven-point hedonic scale. A score ranged from 1 to 7 with the following ratings: 7 = liked extremely, 6 = liked moderately, 5 = liked slightly, 4 = Indifferent, 3 = slightly disliked, 2 = moderately disliked, and 1 = disliked extremely. Unsalted crackers and water were supplied to testers to refresh their palates before tasting subsequent samples.
In an analysis of pH and proximate composition of beef trimmings grade I and II, results were subjected to independent-samples t-tests for comparison of means between the two groups. To study the effects of beef trimmings grade, frozen beef and their interaction, the data were analyzed by the general linear model procedure. Least squares means were computed and separated (p<0.05) using the PDIFF option of GLM. All statistical analyses were performed using SAS v. 9.0 (SAS Inst. Inc., Cary, NC, USA) [20].
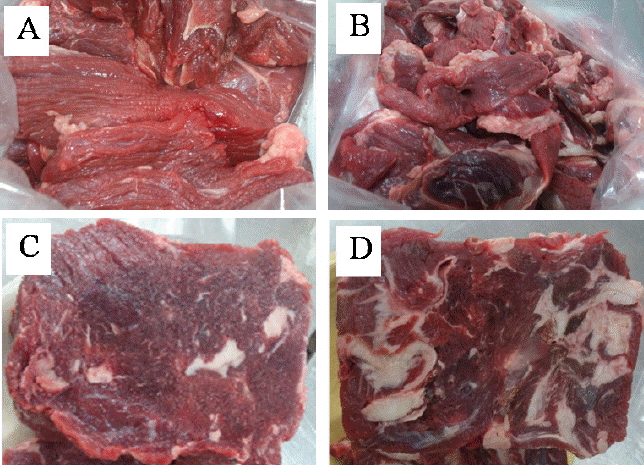
The appearance of beef trimmings grade I and II is shown in Figure 1A and 1B. Among several grades of beef trimmings obtained from a commercial butcher during the manufacture of different primal beef cuts, these collected samples were considered as the top two grades of trimmings by-product due to less visible fat and connective tissue than other trimmings. Chemically, beef trimmings grade I showed higher moisture, protein and ash contents with a lower fat content compared to grade II (p<0.05) (Table 2). Beef trimmings grade I containing 75.65% moisture, 21.16% protein, 2.15% fat, and 1.07% ash. While beef trimmings grade II had 64.52% moisture, 17.59% protein, 16.03% fat, and 0.87% ash.
The pH of beef trimmings grade I was lower than those from grade II (p<0.05) (Table 2), which expressed as 5.7 and 6.0, respectively. The higher pH of beef trimmings grade II rather than grade I might be due to its high-fat content. These results are in agreement with Daszkiewicz et al [21], who reported that high intramuscular fat had a beneficial effect on the pH (high pH) of beef due to an insufficient post-slaughter acidification of meat.
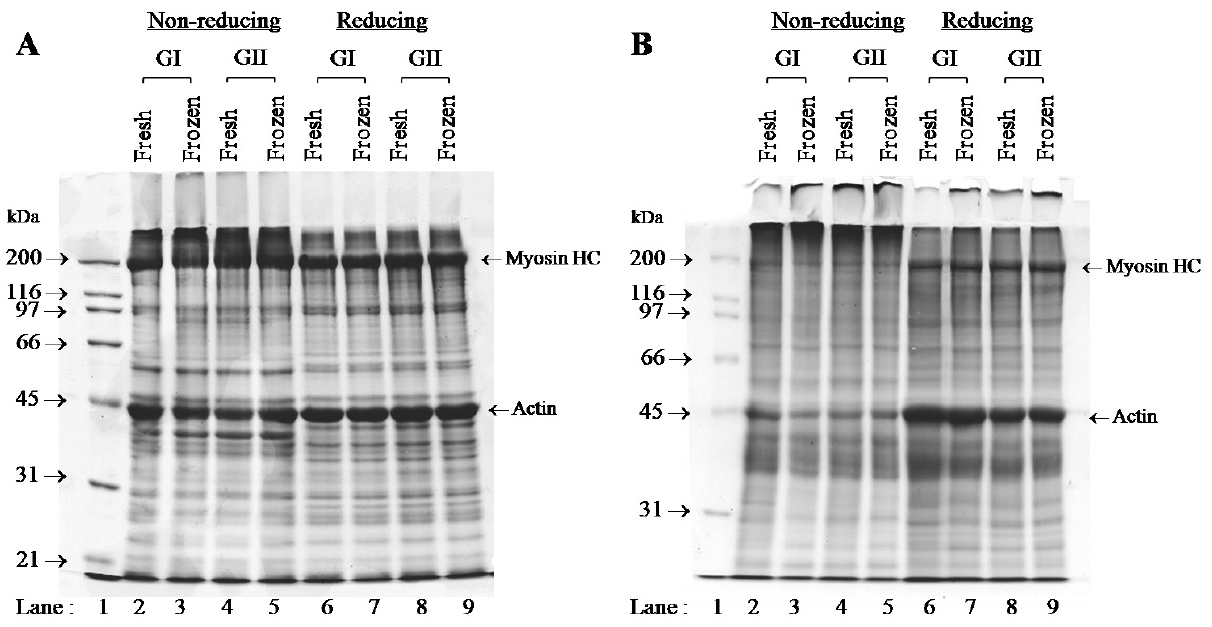
SDS-PAGE protein patterns of raw beef and restructured beef prepared from different treatments are depicted in Figure 2A and 2B. For raw beef samples, without MTGase, the highest band intensity of myosin heavy chain (MHC, 200 kDa) and actin (42 kDa) was found in all beef in both non-reducing and reducing condition (Figure 2A). There were no differences in protein patterns between treatments (beef trimmings grade and frozen beef) either without (Figure 2A) or with MTGase (Figure 2B). However, a SDS-PAGE analysis was a benefit to investigate the potential formation of both fragmented and cross-linked products. Under reducing conditions, reducing agents (such as ╬▓-mercaptoethanol in present study) is incorporated into the SDS-containing loading buffers that reduce disulfide bridges in proteins and then allow protein subunit separation, resulting in new bands appearing after SDS-PAGE [22]. While disulfide-linked proteins are not broken under non-reducing conditions. For the restructured samples in the present study that were separated under non-reducing conditions (Figure 2B, Lane 2ŌĆō5), a high molecular weight protein above 200 kDa and the newly formed protein was observed on the top of resolving gel, while MHC and actin were seen as small and faded bands. On the other hand, regarding Figure 2B, Lane 6ŌĆō9, MHC cloud was partially reproduced (lower intensity as compared to raw beef samples) and actin seemed to be completely reproduced under reducing conditions (same intensity as compared to raw beef samples). These results indicating that MTGase enzyme could induce cross-linking of polypeptide chains through disulfide bonds. Qin et al [23] also reported that the MTGase cross-linking reaction promoted the formation of disulfide bonds of soybean protein isolate and wheat gluten mixture gels. Furthermore, regarding restructured steak, it was noted that under reducing conditions, bands of a high molecular weight protein above 200 kDa and an aggregated protein on the top of resolving gel did not completely disappear. This result suggested that non-disulfide bonds of MHC also participated in stabilizing the reformed beef. Kumazawa et al [24] reported that the intensity of MHC band under reducing condition was reduced gradually as the amount of added MTGase increased, which might be due to non-disulfide covalent cross-linking of the MHC molecules. In surimi gel, MTGase induced the formation of non-disulfide covalent bonds [25], which resulted in the formation of MHC cross-linking and subsequently, a strong gel [26]. As reviewed by Yokoyama et al [27], myosin and actin could be covalently cross-linked by MTGase. Therefore, protein cross-links reaction among restructured beef products with adding MTGase might be supported by both disulfide and non-disulfide bonds of MHC (reducible and non-reducible cross-links under reducing condition, respectively), and also disulfide bond of actin (markedly reducible cross-links under reducing condition) in restructured beef samples. Finally, in the present study, beef trimmings both grade I and II either fresh or frozen could be tightly bound together via cross-linking of MHC and actin, leading to satisfactory obtained restructured products as shown in Figure 1C and Figure 1D, respectively.
Restructured products processed from beef trimmings grade II (belonging high-fat content) showed higher cooking loss value than grade I (Table 3). The results are in agreement with Tornberg et al [28] who concluded that fat was more easily removed during cooking from higher fat beef burgers. Theoretically, weight loss during cooking is due to the losses of both water (and water-soluble components) and fat [29]. From research using microscopy, fat loss from meat products depends on two factors. The first is the instability of the fat itself and the other factor is the ability of the fat to translocate from the inner to the outer parts of the product [29]. In some cases, the solubilization of collagen during cooking may allow melted fat to diffuse along channels. Consequently, this loss occurs due to an expansion of fat droplets as they melt and the formation of pools and channels [29]. The percent of cooking loss increased as the fat level on the ground beef patties increased from 5% to 30% was also reported by Troutt et al [30]. On the contrary, the dense protein matrix of low-fat ground beef prevented fat migration by reducing the probability of fat droplets coalescing and expanding [29]. It is not surprising to find that a higher fat restructured product exhibited the greater cooking loss in present study. Concerning the effect of frozen beef, no significant effect on cooking loss was found (p>0.05). It indicated that the physical damage caused in muscle cells of the frozen sample by ice crystals upon freezing and subsequently thawing was not an important impact on water loss during cooking of restructured beef steaks.
Raw restructured steak from beef trimmings grade I exhibited lower CIE L* (lightness) and CIE b* (yellowness) values with higher CIE a* (redness) than those from beef trimmings grade II (p<0.05) (Table 3). The differences in color among beef trimmings related to a higher content of lean meat which implied a greater myoglobin concentration in beef trimmings grade I than grade II. Furthermore, lightness value of cooked steak was significantly influenced by grade of beef trimmings, where grade I showed increased lightness value than grade II (p<0.05). A more intense discoloration (ΔE) upon cooking was found in beef trimmings grade II than in grade I (p<0.05). Similar results have been reported in beef patties with higher fat content having more intense color changes [31]. These authors explained that a lower heme pigments and the formation of protein oxidation induced by higher lipid content during cooking could affect light reflection and yellowness, leading to color deterioration of beef patties [31]. Although there was no effect of freezing on a color of raw steak, it did have an effect on a cooked steak (p<0.05). Cooked steaks processed from frozen beef had lower redness value than those processed from fresh beef (p<0.05). Denaturation of the globin moiety of the myoglobin molecule takes place during freeze-thaw process, leading to an increased susceptibility of myoglobin to autoxidation and subsequent loss of optimum color presentation [32].
There were no significant differences in shear force of raw restructured steaks among treatments (p>0.05) (Table 3). Variations in the tenderness of cooked beef steaks as indicated by shear force was largely affected by freezing in which the product from frozen beef showed lower shear force than fresh beef (p<0.05). Tenderness of raw meat increased with increasing number of freeze-thaw cycles, which related to the loss of structural integrity caused by ice crystal formation and small intracellular ice crystals and probably by the release of protease enzymes [32]. Generally, WBSF refers to the maximum force required to shear through a sample and used as an index of meat toughness. Moreover, Canto et al [33] evaluated the binding properties of restructured caiman steaks containing MTGase using TPA analysis results. In present study, the effect of freezing on TPA was also more pronounced in raw steaks than in cooked steak (Table 3). Raw steak made from frozen beef showed lower hardness, gumminess, and chewiness, but higher cohesiveness and springiness than those made from fresh beef (p<0.05), representing a softer texture but a stronger meat binding. Canto et al [33] and Herroero et al [34] reported that the increase in springiness and cohesiveness observed in meat systems containing MTGase can be attributed to the enhanced protein cross-linking between meat particles. Regarding the effect of beef trimmings grade, it largely impacted on hardness among beef samples. Hardness of raw steak processed from beef trimmings grade II tended to lower (p<0.07) and hardness of its cooked steak was lower (p<0.05) as compared to beef trimmings grade I. It could be due to the fact that a higher fat level in beef trimmings grade II. The deposition of fat, either intramuscular fat, intrafasicular or intracellular fat, tends to provide a more tender and potentiates the sensation of the tenderness of meat and meat products [29].
Although there were no significant differences in microbial counts among raw restructured steaks from different treatments, they showed viable counts determined on PCA ranging from 4.72 to 5.40 Log CFU/g for psychrotrophic bacteria and from 3.38 to 3.91 Log CFU/g for mesophilic bacteria depending on treatments, while thermophilic bacterial counts showed a below the detection level (Table 4). The number of psychrophilic bacteria was, on average, 1 log cycle higher than mesophilic bacteria in each sample. These results are in agreement with those reported by Ercolini et al [35] in refrigerated beef. They stated that bacteria developing on meat at chill temperatures are regarded as psychrotrophic populations belonging to microbial genera of both gram-positive, such as lactic acid bacteria, and Gram-negative bacteria, such as Pseudomonas spp. and Enterobacteriaceae [36]. Species of Pseudomonas are particularly involved in the spoilage of meat stored at chill temperatures [37]. After grilling the steaks until the CT reached 71┬░C, psychrotrophic and mesophilic bacteria counts of all samples were reduced to an undetectable level.
Beef trimmings grade influenced some sensory attributes as shown in Table 5. Restructured beef made from beef trimmings grade II exhibited a higher sensory score of tenderness (p<0.05) as well as tending towards higher scores of juiciness and overall acceptability (p<0.07) compared with those from sample grade I. Berry et al [38] found that the steaks with higher fat levels (18% and 22%) were juicier, moister and had greater mouth coating property than lower fat level (10% and 14%). Moreover, Iida et al [39] reported that an increase in fat content of cooked beef increased the tenderness, juiciness, and fattiness scores and also enhanced the umami intensity and beef flavor intensity, leading to an improved overall evaluation score. The eating satisfaction of beef usually results from a combination of tenderness, juiciness and flavor [40]. According to present study, the sensory evaluation showed no significant differences among sensory attributes between the fresh and frozen meat (p<0.05). This meant that the processing could use the meat that has been frozen and then thawed for 1 cycle, which would contribute flexibility to process for industry. The results are in agreement with Rahman et al [41], who observed that the deterioration in sensorial quality was small and significant only when the freeze-thaw cycle was repeated after two or three cycles.
Beef trimmings grade II with possessing high-fat level (16.03% fat) could be a suitable raw material for processing restructured beef steaks. Although some detrimental effect on cooking loss and discoloration after cooking was observed, higher scores of tenderness, juiciness and overall acceptability were found in restructured steaks processed from beef trimmings grade II than from those made from beef trimmings grade I (2.15% fat). Freezing of beef trimmings could improve the meat binding and soft texture of restructured steak as evaluated by instrumental texture analysis without negatively affecting sensory attributes, allowing flexibility to meat producers.
ACKNOWLEDGMENTS
The authors would like to express their sincere thank to Faculty of Agricultural Technology, KMITL for the financial support, and undergraduate student, Panita Jungwarawit and Wanchalerm Phruksawan for some laboratory assistant.
Figure┬Ā1
Beef trimmings grade I (A) and grade II (B). Obtained raw restructured steaks from beef trimmings grade I (C) and grade II (D).
Figure┬Ā2
Electrophoretic patterns of muscle protein from raw beef (A) and raw restructured beef steaks containing microbial transglutaminase (B) separated by 10% running gel. GI, Beef trimmings grade I; GII, Beef trimmings grade II.
Table┬Ā1
Treatment formulation of restructured steaks
Table┬Ā2
Proximate composition and pH of beef trimmings from different grades
| Parameters | Beef trimmings | |
|---|---|---|
|
|
||
| Grade I | Grade II | |
| Moisture (%) | 75.65┬▒0.08a,1) | 64.52┬▒2.09b |
| Protein (%) | 21.16┬▒0.02a | 17.59┬▒0.34b |
| Fat (%) | 2.15┬▒0.30b | 16.03┬▒0.26a |
| Ash (% | 1.07┬▒0.02a | 0.87┬▒0.01b |
| pH | 5.70┬▒0.20b | 6.00┬▒0.10a |
Table┬Ā3
Physical characteristics of raw and cooked restructured beef steaks containing MTGase
| Parameters | Beef trimmings | Freezing | SEM | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Grade I | Grade II | p-value1) | Fresh | Frozen | p-value1) | ||
| Cooking loss | 32.242) | 37.74 | 0.002 | 34.95 | 35.03 | 0.948 | 0.85 |
| Raw attributes | |||||||
| ŌĆāCIE L* | 38.66 | 46.36 | <0.001 | 42.12 | 43.91 | 0.058 | 0.60 |
| ŌĆāCIE a* | 17.55 | 16.42 | 0.034 | 16.99 | 16.97 | 0.958 | 0.25 |
| ŌĆāCIE b* | 15.10 | 15.58 | 0.045 | 15.17 | 15.52 | 0.107 | 0.12 |
| ŌĆāShear force (N) | 4.69 | 5.07 | 0.382 | 5.20 | 4.56 | 0.153 | 0.31 |
| ŌĆāHardness (N) | 3.14 | 2.65 | 0.061 | 3.30 | 2.49 | 0.004 | 0.19 |
| ŌĆāCohesiveness (ratio) | 0.50 | 0.52 | 0.349 | 0.48 | 0.53 | 0.043 | 0.01 |
| ŌĆāGumminess (N) | 1.63 | 1.44 | 0.110 | 1.92 | 1.15 | <0.001 | 0.11 |
| ŌĆāSpringiness (ratio) | 0.91 | 0.92 | 0.094 | 0.90 | 0.93 | <0.001 | 0.01 |
| ŌĆāChewiness (N) | 1.60 | 1.22 | 0.006 | 1.68 | 1.14 | <0.001 | 0.09 |
| Cooked attributes | |||||||
| ŌĆāCIE L* | 34.37 | 30.42 | 0.012 | 31.16 | 33.64 | 0.077 | 0.86 |
| ŌĆāCIE a* | 8.28 | 8.82 | 0.111 | 9.37 | 7.73 | <0.001 | 0.21 |
| ŌĆāCIE b* | 22.32 | 21.69 | 0.585 | 22.24 | 21.77 | 0.685 | 0.78 |
| ŌĆāColor deference (╬öE)3) | 12.88 | 19.38 | 0.002 | 16.53 | 15.73 | 0.444 | 0.66 |
| ŌĆāShear force (N) | 5.60 | 5.09 | 0.318 | 6.41 | 4.27 | <0.001 | 0.34 |
| ŌĆāHardness (N) | 23.32 | 19.29 | 0.023 | 22.91 | 19.70 | 0.063 | 1.17 |
| ŌĆāCohesiveness (ratio) | 0.62 | 0.64 | 0.141 | 0.62 | 0.64 | 0.293 | 0.01 |
| ŌĆāGumminess (N) | 11.40 | 13.19 | 0.055 | 12.60 | 11.99 | 0.496 | 0.62 |
| ŌĆāSpringiness (ratio) | 0.91 | 0.92 | 0.273 | 0.91 | 0.91 | 0.614 | 0.01 |
| ŌĆāChewiness (N) | 12.68 | 12.10 | 0.521 | 12.72 | 12.06 | 0.470 | 0.65 |
Table┬Ā4
Microbial counts (Log CFU/g) of restructured beef steaks containing MTGase from different beef trimmings grade and freezing
| Microbial count | Beef trimmings | Freezing | SEM | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Grade I | Grade II | p-value1) | Fresh | Frozen | p-value1) | ||
| Raw samples | |||||||
| ŌĆāPsychrotrophic | 4.722) | 5.40 | 0.071 | 5.18 | 4.84 | 0.093 | 0.093 |
| ŌĆāMesophile | 3.91 | 3.38 | 0.107 | 3.73 | 3.55 | 0.514 | 0.514 |
| ŌĆāThermophile | ND3) | ND | - | ND | ND | - | - |
| Cooked samples | |||||||
| ŌĆāPsychrotrophic | ND | ND | - | ND | ND | - | - |
| ŌĆāMesophile | ND | ND | - | ND | ND | - | - |
| ŌĆāThermophile | ND | ND | - | ND | ND | - | - |
Table┬Ā5
Sensory attributes of cooked restructured beef steaks containing MTGase as influenced by beef trimmings grade and freezing
| Attributes | Beef trimmings | Freezing | SEM | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Grade I | Grade II | p-value1) | Fresh | Frozen | p-value1) | ||
| Color | 4.202) | 4.41 | 0.316 | 4.37 | 4.25 | 0.589 | 0.15 |
| Appearance | 4.38 | 4.38 | 1.000 | 4.50 | 4.26 | 0.232 | 0.20 |
| Flavor | 4.26 | 4.23 | 0.882 | 4.30 | 4.20 | 0.657 | 0.16 |
| Tenderness | 3.95 | 4.48 | 0.049 | 4.22 | 4.17 | 0.839 | 0.17 |
| Juiciness | 3.70 | 4.35 | 0.064 | 4.03 | 3.87 | 0.277 | 0.17 |
| Overall acceptability | 4.10 | 4.46 | 0.068 | 4.29 | 4.19 | 0.576 | 0.12 |
REFERENCES
1. Office of Agricultural Economic. Situation of agricultural products and trends in Thailand [Internet]. Office of Agricultural Economic; 2015. [cited 2015 Feb 28]. Available from: http://www.oae.go.th/download/document_tendency/journalofecon2558.pdf
2. Kuraishi C, Sakamoto J, Yamazaki K, et al. Production of restructured meat using microbial transglutaminase without salt or cooking. J Food Sci 1997; 62:488ŌĆō90.

3. Lee HG, Lanier TC. The role of covalent cross-linking in the texturizing of muscle protein sols. J Muscle Foods 1995; 6:125ŌĆō38.

4. Antequera T, P├®rez-Palacios T, Rodas E, Rodr├Łguez M, C├│rdoba JJ. Effect of muscle type and frozen storage on the quality parameters of Iberian restructured meat preparations. Food Sci Technol Int 2014; 20:543ŌĆō54.


5. Sen AR, Karim SA. Effect of meat particle size on quality attributes of restructured mutton steaks. J Food Sci Technol 2003; 40:423ŌĆō5.
6. Farouk MM, Zhang SX, Cummings T. Effect of muscle fiber/fiber-bundle alignment on physical and sensory properties of restructured beef steaks. J Muscle Food 2005; 16:256ŌĆō73.

7. Farouk MM, Hall WK, Wieliczko KJ, Swan JE. Processing time and binder effect on the quality of restructured rolls from hot-boned beef. J Muscle Food 2005; 16:318ŌĆō29.

8. Gadekar YP, Sharma BD, Shinde AK, Mendiratta SK. Restructured meat products - production, processing and marketing: A review. Indain J Small Rumin 2015; 21:1ŌĆō12.

9. Heinz G, Hautzinger P. Meat processing technology for small-to medium-scale producers [Internet]. Bangkok: Food and Agriculture Organization of the United Nations Regional Office for Asia and Pacific; 2007. [cited 2015 Feb 28]. Available from: http://www.fao.org/docrep/010/ai407e/AI407E05.htm
10. Sethakul J, Sivapirunthep P. Meat processing. Bangkok, Thailand: Mean Service Supply Ltd., Part; 2012.
11. Savell JW, Branson RE, Cross HR, et al. National consumer retail beef study: Palatability evaluations of beef loin steaks that differed in marbling. J Food Sci 1987; 52:517ŌĆō9.

12. Neely TR, Lorenzen CL, Miller RK, et al. Beef customer satisfaction: Role of cut, USDA quality grade, and city on in-home consumer ratings. J Anim Sci 1998; 76:1027ŌĆō33.


13. Wheeler TL, Miller RK, Savell JW, Cross HR. Palatability of chilled and frozen beef steaks. J Food Sci 1990; 55:301ŌĆō3.

14. Lagerstedt A, Enf├żlt L, Johansson L, Lundstr├Čm K. Effect of freezing on sensory quality, shear force and water loss in beef M. longissimus dorsi
. Meat Sci 2008; 80:457ŌĆō61.


15. Latimer GW; AOAC International. Official methods of AOAC International. 19th edGaithersburg, MD, USA: AOAC International; 2012.
16. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage. Nature 1970; 227:680ŌĆō5.


17. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265ŌĆō75.


18. Bourne MC. Texture profile analysis. Food Technol 1978; 32:62ŌĆō5.
19. Downes FP, Ito K. Compendium of methods for the microbiological examination of foods. 4th edWashington, DC, USA: American Public Health Association; 2001.
20. SAS Institute Inc. SAS Version 9.0. Cary, NC, USA: SAS Institute; 2002.
21. Daszkiewicz T, Wajda S, Kondratowicz J. Physico-chemical and sensory properties of meat from Black-and-White and Black-and-White├Ś Limousine heifers differing in intramuscular fat content. Anim Sci Pap Rep 2005; 23:181ŌĆō7.
22. Corley RB. A guide to methods in the biomedical sciences. New York, NY, USA: Springer Science+Business Media, Inc.; 2005.
23. Qin XS, Luo SZ, Cai J, et al. Effects of microwave pretreatment and transglutaminase crosslinking on the gelation properties of soybean protein isolate and wheat gluten mixtures. J Sci Food Agric 2016; 96:3559ŌĆō66.


24. Kumazawa Y, Seguro K, Takamura M, Motoki M. Formation of ╔ø-(╬│-glutamyl) lysine cross-link in cured horse mackerel meat induced by drying. J Food Sci 1993; 58:1062ŌĆō4.

25. Tammatinna A, Benjakul S, Visessanguan W, Tanaka M. Gelling properties of white shrimp (Penaeus vannamei) meat as influenced by setting condition and microbial tranglutaminase. LWT Food Sci Technol 2007; 40:1489ŌĆō97.

26. Chaijan M, Panpipat W. Gel-forming ability of mackerel (Rastrelliger Branchysoma) protein isolate as affected by microbial transglutaminas. Walailak J Sci Technol 2010; 7:41ŌĆō9.
27. Yokoyama K, Nio N, Kikuchi Y. Properties and applications of microbial transglutaminase. Appl Microbiol Biotechnol 2004; 64:447ŌĆō54.


28. Tornberg E, Olsson A, Persson K. A comparison in fat holding between hamburgers and emulsion sausages. In : Proceeding of the 35th International Congress of Meat Science and Technology; 1989. 1989 August 20ŌĆō25; Copenhagen, Denmark. p. 752ŌĆō9.
29. Desmond E, Troy DJ. Nutrient claims on packaging. Jensen WK, Devine C, Dikeman M, editorsEncyclopedia of meat sciences. Oxford, UK: Elsevier; 2004. p. 903ŌĆō9.

30. Troutt ES, Hunt MC, Johnson DE, et al. Chemical, physical and sensory characterisation of ground beef containing 5 to 30 per cent fat. J Food Sci 1992; 57:25ŌĆō9.

31. Utrera M, Morcuende D, Est├®vez M. Fat content has a significant impact on protein oxidation occurred during frozen storage of beef patties. LWT Food Sci Technol 2014; 56:62ŌĆō8.

32. Leygonie C, Britz TJ, Hoffman LC. Impact of freezing and thawing on the quality of meat: review. Meat Sci 2012; 91:93ŌĆō8.


33. Canto ACVCS, Costa Lima BRC, Suman SP, et al. Physico-chemical and sensory attributes of low-sodium restructured caiman steaks containing microbial transglutaminase and salt replacers. Meat Sci 2014; 96:623ŌĆō32.


34. Herrero AM, Cambero MI, Ordonez JA, de la Hoz L, Carmona P. Raman spectroscopy study of the structural effect of microbial transglutaminase on meat systems and its relationship with textural characteristics. Food Chem 2008; 109:25ŌĆō32.


35. Ercolini D, Russo F, Nasi A, Ferranti P, Villani F. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl Environ Microbiol 2009; 75:1990ŌĆō2001.



36. Gill CO, Newton KG. The ecology of bacterial spoilage of fresh meat at chill temperatures. Meat Sci 1978; 2:207ŌĆō17.


37. Jay JM, Vilai JP, Hughes ME. Profile and activity of the bacterial biota of ground beef held from freshness to spoilage at 5ŌĆō7┬░C. Inter J Food Microbiol 2003; 81:105ŌĆō11.

38. Berry BW, Smith JJ, Secrist JL. Effects of fat level on sensory, cooking and instron properties of restructured beef steaks. J Anim Sci 1985; 60:434ŌĆō9.

39. Iida F, Saitou K, Kawamura T, Yamaguchi S, Nishimura T. Effect of fat content on sensory characteristics of marbled beef from Japanese Black steers. Anim Sci J 2015; 86:707ŌĆō15.


- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





