 |
 |
| Anim Biosci > Volume 29(12); 2016 > Article |
|
Abstract
This study was conducted to examine the effects of adding phytoncide oil extracted from Korean pine nut cone byproduct to the diet of dairy cows on milk yield and compositions, fatty acid characteristics, complete blood count and stress response. A total of 74 Holstein cows were used for 30 days and divided into two groups. Each group was given a basal diet (C) or an experimental diet containing phytoncide additives at 0.016% (T) in feed. The results showed that phytoncide feeding had no effect on milk yield. In addition, there were no observed effects on milk composition, but the ratio of fatty acid in milk was significantly affected by the phytoncide diet, and it showed a positive effect. Not only were the major functional fatty acids, conjugated linoleic acid and eicosapentaenoic acid increased, but also Žē6:Žē3 fatty acid ratio was reduced in milk of T group (p<0.05). In blood analysis, the complete blood count showed no significant difference between C and T group on all parameters. However, the cortisol concentration was significantly decreased in T group compared to control (p<0.05). Taken together, we suggest that phytoncide oil does not have a great influence on the physiological changes, but can be a potential feed additive that improves the milk fatty acid and stress resilience in dairy cows. In addition, it will contribute to the development of feed resource, a reduction in feed cost and a lessening of environmental pollution.
The use of antibiotic growth promoters has proven to be a useful means of improving feed efficiency (Yang et al., 2007). However, the usage of antibiotics has already been banned. Accordingly, both locally and abroad, substances that can be used as substitutes for a number of antibiotics have been developed. Among the various alternatives, the use of phytogenic materials in livestock production has greatly increased. We have chosen a natural material for our study. Phytoncide or essential oil is a natural substance and a food by-product derived from Korean origin pine nut cone. Currently, South Korea produces 2,435 tons of pine nuts annually, which are widely distributed to other Asian countries such as Japan and China. After pine nut cones are harvested, only nuts are used as the edible part, and the other by-products thrown away. As this waste by-product might cause environmental pollution, the utilization of discarded pine by-product extract is an efficient use of resources that would have been wasted otherwise.
In addition, pine resin contains terpenoid, phenols, tannins and alkaloids components. These secondary metabolites are materials produced from specific metabolism in certain species, which have antibacterial and insecticidal effects (Abe et al., 2008). Due to its various biological properties, this natural product is considered suitable for use as an alternative material.
Essential oils are complex mixtures of secondary metabolites and volatile compounds extracted from plants through distillation methods. Such plant-derived essential oils are means of improving the feed efficiency and health of dairy cattle (Yang et al., 2007). Plant extracts stimulate the immune system, thereby enhancing the resistance to inflammatory and infectious diseases. The study of Concha et al. (1996) showed that ginseng root extract stimulated neutrophils and lymphocytes from bovine peripheral blood and milk (Yang et al., 2007). Also, phytoncide exposure decreased stress hormone levels, because it also contributed to the activity of natural killer (NK) cells, a type of cytotoxic lymphocyte (Li, 2010).
Some studies have investigated the effects of essential oil or their components on digestion, ruminal fermentation, milk composition and milk production in dairy cows (Benchaar et al., 2007). As well, Benchaar et al. (2007) reported a change in the milk fatty acid profile when cows were supplemented daily with a mixture of essential oil compounds. Supplementing the mixture at a higher concentration increased the concentration of conjugated linoleic acid (CLA), a health-promoting fatty acid in milk fat (Benchaar et al., 2006). Among the different effective ingredients of milk, CLA is particularly important, as it has many positive health effects including anti-tumor effects (Igal, 2011), anti-atherosclerosis effects (Lee and Vanden Heuvel, 2010) and anti-diabetic effects (Moloney et al., 2007).
However, knowledge is still rather limited regarding the effects of pine-derived phytoncide on livestock. Therefore, the objective of this study was to investigate the effects of phytoncide extracted from discarded Korean pine nut cones on milk fatty acid composition and mitigation of stress in dairy cows.
To conduct the feeding experiment, a total of 74 Holsteins (mean 35 kg milk yield, 644 day lactation cycle and 2.5 calving number) were randomly assigned to two groups and studied for 30 days. All experimental procedures involving animals were performed according to the Animal Experimental Guidelines provided by the Animal Care and Use Committee of Konkuk University, Republic of Korea.
Dairy cows were fed total mixed ration (86.9%), roughage (3.9%), and concentrated feed (9.2%). The experimental diets were formulated to meet or exceed the NRC recommendations (NRC, 2001) and were provided for ad libitum intake. Ingredient and chemical composition of experimental diet are presented in Table 1. Equal portions of feed and water were given to cows, and the diet was available at all times.
We received a phytoncide oil preparation from PHYLUS Co., Ltd. Essential oil was obtained by water-vapor methods. Briefly after removing the pine nuts, pine nut cones were dried in the shade and the by-products were cut in 2 to 3 cm size. Pine nut cones were added into a 1,000 mL round flask and homogenized with distilled water (0.5 g/500 mL of water) for 1 to 2 min, and distilled by steam distillation process (100┬░C┬▒3┬░C, 3 h). After cooling the extract (20┬░C), the pine nut cones extract (phytoncide essential oil) was separated from water using the difference in specific gravity. Then, the extract was filtrated and concentrated (60┬░C, 100 mHg). Finally, the extract was sealed and stored at 4┬░C.
The chemical composition of phytoncide oil was analyzed using Gas Chromatograph/Mass Spectrometer (6890N series GC/MSD System, Agilent Technologies, Palo Alto, CA, USA). The analysis conditions are as follows.
Phenomenex Zebron ZB-5ms (dimension: 30 meter├Ś0.25 mm├Ś0.25 ╬╝m); injection: split 50:1, heater 255┬░C, injection volume 1.0 ╬╝L, carrier gas: helium 1.0 mL/min; oven program: 40┬░C to 300┬░C, 5/min; detector: MS System, heater 280┬░C.
The main components of the essential oil were gamma-Terpinene (26.8%), dl-Limonene (19.6%), beta-Pinene (16.2%), and Isolongifolene (4.2%) (Table 2). Phytoncide feed additive consists of rice bran 60% and corn grit 40% as excipients. Phytoncide oil was mixed with the vehicle at a 10% concentration and it was added to the basal diet at a final concentration of 0.016% by top dressing. The control group was fed the same amount of added the vehicle (not included phytoncide oil).
During the experimental period, we collected milk twice a day, at 4 am and at 4 pm. The total milk yield was then calculated, and the mean for 10 days was measured. At the end of the period, average milk yield was calculated. After mixing the collected milk in the morning and afternoon, the samples were stored at 4┬░C for fatty acid analysis. Milk composition was measured with MilkoScan (CombiFoss FT+500 S/H, Hiller├Ėd, Denmark). Levels of milk fat, milk protein, solid-not-fat (SNF), somatic cells, milk urea nitrogen (MUN), and beta-hydroxybutyrate were analyzed at 0 d, 10 d, 20 d, and 30 d.
Feed samples were analyzed for dry matter (DM), crude protein, ether extract, crude fiber, crude ash, neutral detergent fiber (NDF) and acid detergent fiber (ADF) according to AOAC (1990) procedures. DM content was determined by drying samples in a vacuum oven at 100┬░C overnight. Ash content was determined by incineration at 550┬░C overnight in a muffle furnace. Crude protein (N├Ś6.25) was determined using the Kjeltec System (Kjeltec 2400, FOSS, Hiller├Ėd, Denmark). Crude fiber, NDF and ADF content of the feed samples were analyzed using the Fibertec Systems (Fibertec 2010, FOSS, Denmark). Ether extract contents were obtained using the ether extraction system (ANKOM XT15 Extractor, ANKOM Technology, NY, USA), and mineral contents of the feed were determined by inductively coupled plasma optical emission spectrometry (ICP-OES, Thermo, Waltham, MA, USA).
Lipid was extracted using chloroform and methanol (Folch et al., 1957). FAME Mix STANDARD (Sigma-Aldrich/47885-U, St. Louis, MO, USA) was the standard for fatty acid content and fatty acid ratio. The measurement equipment was Gas Chromatograph/FID (HP 6890 series GC System, Agilent technologies, USA), and the analysis conditions are as follows.
Sp-2560 Capillary column (dimension: 100 meter├Ś0.25 mm├Ś0.2 ╬╝m, film thickness); injection: split 30:1, heater 255┬░C, pressure: 32.64, total flow: 39.5 mL/min, split flow: 36.0 mL/min, injection volume 1.0 ╬╝L, carrier gas: helium 1.2 mL/min; oven program: 70┬░C to 100┬░C: 5/min (Hold: 2min), 100┬░C to 175┬░C: 10/min (hold 40 min), 175┬░C to 225┬░C: 5/min (hold 40 min); detector: FID System, heater 260┬░C (H2 flow: 40 mL/min, air flow: 400 mL/min).
At the end of the feeding period, 3 hours after feeding, 10 cows were selected from each group according to their milk yield, milk compositions, lactation cycle and calving number. Blood sampling was carried out from the jugular vein of cows. The whole blood was subjected to complete blood count (CBC) test using the HM2 (VetScan HM2 Hematology System, Abaxis, Union City, CA, USA). In addition, cortisol levels were measured with commercially available Bovine Cortisol ELISA Test kit (ENDOCRINE TECHNOLOGIES, Inc., Newark, CA, USA) according to the manufacturerŌĆÖs instructions.
The JMP 7.0 (SAS Institute, Cary, NC, USA) program was used for all statistical analyses. Each result is expressed as the mean┬▒standard error of the mean. Comparisons between two groups and multiple groups were evaluated using StudentŌĆÖs t-test. A probability of less than 0.05 was considered statistically significant.
Numerous studies were conducted on the effects of essential oil (EO) on rumen microbial fermentation, milk production, and milk composition of dairy cows (e.g., Benchaar et al., 2006; 2007; Yang et al., 2007). The general range of EO was about 0.01% to 0.04% of the feed. Study by Benchaar et al. (2007) reported that supplementing the EO mixture at 0.01% concentration (i.e., 2 g/d) increased the amount of CLA a health-promoting fatty acid in milk. Moreover, the study of Khiaosa-ard and Zebeli (2013) on rumen fermentation variables suggested that EO affected protozoa numbers (p<0.001) but only high doses of 0.02% of DM had an inhibitory effect on this variable whereas lower doses promoted the number. In this regard, we thought that the appropriate range of EO concentration was 0.01% to 0.02%. In fact, we found that 0.008% of the same EO supplement contributed to promotion of CLA in egg yolk in our previous experiment. Based on our previous study, we decided to use double concentration (0.016%) that also is between 0.01% and 0.02%.
The average feed intake of cows was 37.31 kg/d. Average DM intake was 23.59 kg/d and did not differ for cows fed diets containing phytoncide additive. In addition, there were no remaining feed.
During the 30-day experimental period, no differences between the groups were shown in terms of changes in daily milk yield (p>0.05). Milk yield average was 34.3 kg/d, and was not affected by additives (Table 3). Milk compositions were analyzed at 0 d, 10 d, 20 d, and 30 d, but there were no differences. Average milk fat and protein concentrations were 4.2% and 3.1%, respectively, and were unaffected by phytoncide additive (Table 4). In addition, there were no effects on SNF, somatic cells, MUN, beta-hydroxybutylate and acetone. The 30-day average lactose (lactose) was increased in comparison with the control (p<0.05), but the small change in the amount of lactose is within the normal range. Therefore, the phytoncide feeding range is not believed to induce a great influence in terms of physiological changes of the cow.
Similarly, supplementation of dairy cows with peppermint at 20 g/kg DM had no effect on milk yield and milk composition (Hosoda et al., 2005). A study by Yang et al. (2007) reported that the addition of garlic and juniper berry essential oils to dairy cow diets had no effect on DM intake, milk production or milk composition. In addition, there was no effect on milk performance observed when cows were supplemented with a mixture of EO. This was because these plant extracts did not have any effect on feed intake and ruminal fermentation (Benchaar et al., 2008). Therefore, additive modification of the level and adjustment of feeding term of EO is needed to assess further in vivo experiment.
Overall, phytoncide supplement in dairy cows showed a significant effect on the milk fatty acid profile (Table 5). Phytoncide treatment increased the milk fat contents of cis-9, trans-11 and trans-10, cis-12-CLA compared with the control (p<0.05). The CLA are a family of at least 28 isomers of linoleic acid generally found in the meat and dairy products derived from ruminants. Food products from grass-fed ruminants are good sources of CLA (Dhiman et al., 2000). Healthy functional fatty acid CLA is a material with diverse physiological activities. It has been attributed with many beneficial effects in the prevention of atherosclerosis, different types of cancer and hypertension, and is also known to improve immune function (Bhattacharya et al., 2006). Benchaar et al. (2007) reported no change in milk fatty acid profile when cows were supplemented daily with 750 mg of mixture of essential oil. However, supplementing the same mixture at a higher concentration (i.e., 2 g/d) increased the concentration of CLA, a health-promoting fatty acid, in milk fat (Benchaar et al., 2006). In our trial, though a small amount was added to the feed, CLA content of the milk was increased.
Among the different rumen bacteria, Butyrivibrio fibrisolvens can produce significant amounts of cis-9, trans-11 CLA from the linoleic acid in rumen, and cis-9, trans-11 CLA is transported to the mammary glands (Kim et al., 2000; Wang and Lee, 2015). CLAs produced in the rumen are significantly affected by diet. Linoleic acid is a major source of CLA, which is mostly found in plant oil. Thus, supplying these oilseeds can improve cis-9, trans-11 CLA content in milk (Wang and Lee, 2015). Furthermore, CLA isomers in the rumen are synthesized through various mechanisms. The other cis-9, trans-11 CLA is de novo synthesized from trans-11 vaccenic acid (TVA) in the mammary gland (Griinari et al., 2000; Corl et al., 2001; Kay et al., 2004). Stearoyl-CoA desaturase (Δ9-desaturase) is an important enzyme that plays a key role in cis-9, trans-11 CLA synthesis from TVA in the mammary gland (Griinari et al., 2000; Corl et al., 2001; Kay et al., 2004; Wang and Lee, 2015). Therefore, additional research on the synthesis of CLA in the rumen and mammary gland by phytoncide is required.
In addition, eicosapentaenoic acid (EPA) content of the phytoncide treated group was significantly higher than that of the control. Accordingly, Žē6/Žē3 fatty acid ratio was decreased (p<0.05) (Table 5). EPA is a precursor of docosahexaenoic acid (DHA), and DHA is one of the fatty acids of special interest because of its importance in brain and retinal neonatal development (Connor, 2000). The ratio of Žē6/Žē3 fatty acids has a valuable influence on dairy products because a low Žē6/Žē3 ratio is more desirable in reducing the risk of many chronic diseases (Simopoulos, 2010; Abarghuei et al., 2014). Abarghuei et al. (2014) showed that the addition of pomegranate-peel extract decreased Žē6/Žē3 ratio in cowŌĆÖs milk. The increase of the long-chain Žē-3 fatty acid such as EPA, DHA could be due to desaturation and elongation of ╬▒-linolenic acid (ALA, C18:3n3). ALA and EPA converted to its elongated metabolite DHA, and then deposited it in the milk. Given that humans seem to have a limited ability to desaturate the last step of the formation from ALA, milk can be considered a useful dietary source of such fatty acids (Baucells et al., 2000; Carrillo-Dominguez et al., 2005).
In general, essential oil has an antibacterial activity against gram-negative and positive bacteria (Helander et al., 1998). Several gram bacteria are involved in the ruminal bio-hydrogenation of unsaturated dietary fatty acids (Harfoot and Hazlewood, 1997). Therefore, feeding essential oil could lower the number and the activity of rumen bacteria involved in the bio-hydrogenation of unsaturated fatty acids. This means that the inhibition of ruminal bio-hydrogenation or ruminal bacteria activity by phytoncide metabolites could have produced higher unsaturated fatty acids from rumen to milk.
In addition, saturated fatty acid, C12:0 were lower in the milk of phytoncide treated group compared with the control (p<0.05). Similarly, the reduction of saturated fatty acid in milk is very helpful to the health of humans who drink milk, because dietary saturated fat intake has been associated with an increased risk of cardiovascular disease (Siri-Tarino et al., 2010). These results are considered to indicate that the phytoncide feed additives may provide benefits for the production of milk, and would ultimately promote a better fatty acid composition for the human diet.
The result of CBC analysis in whole blood is presented in Table 6. Phytoncide treatment did not affect white blood cells, red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin or mean corpuscular hemoglobin concentration compared to the control group. This result is considered to indicate that feed with added phytoncide does not induce any disease-related chemical changes in dairy cows.
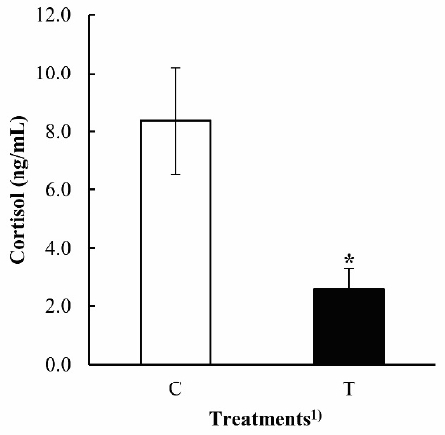
In blood analysis, cortisol levels of the group that received phytoncide treatment were significantly decreased compared to the control in dairy cows after 30 days (p<0.05) (Figure 1). The normal range of blood cortisol concentration is 1 to 17 ng/mL in dairy cows (Lefcourt et al., 1993). In our first test, cortisol concentration averaged 6.2┬▒2.13 ng/mL (C) and 7.2┬▒2.05 ng/mL (T), and then 8.4┬▒1.83 ng/mL (C) and 2.6┬▒0.74 ng/mL (T) after experiment. Values did not exceed basal cortisol levels. Cortisol has been used mainly as a key indicator of the stress response in cows. A high cortisol level may produce negative effects and may down-regulate some functions such as eating, growth, and reproduction (Burnett et al., 2014). Chrousos (2009) reported that elevated cortisol concentration is a risk factor for chronic stress that can cause additional behavioral and somatic disorders. In our research, it was found that phytoncide relieved the negative effects of plasma cortisol in cows. These findings indicate that a phytoncide supplement decreased stress hormone levels, which may partially contribute to immune activity (Li, 2010). Ultimately, this will lead to more optimal productivity and health of dairy cows.
The results of this study showed that feeding of essential oils had no effect on milk yield and composition, and thus it is determined that phytoncide will not cause significant physiological changes in cows. However, positive effects were seen in the fatty acids content in milk, as the functional fatty acids (CLA and EPA) and Žē3/Žē6 ratio were increased in the treated group. Furthermore, cortisol, a stress hormone, significantly decreased in serum of cows fed phytoncide additives. Taken together, these data indicate that phytoncide is a potential feed additive for improving milk fatty acid and promoting healthy functional milk production in dairy cows.
ACKNOWLEDGMENTS
This research was supported by the Agricultural Biotechnology Development Program (313120-04), Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Figure┬Ā1
Effect of dietary phytoncide on level of serum cortisol in dairy cow. Values are expressed as mean┬▒standard error of the mean (n = 10), * p<0.05 by StudentŌĆÖs t-test. 1 C, control; T, phytoncide (0.016%/feed).
Table┬Ā1
Ingredient and chemical composition of the experimental diet
| Item | Diets | |||
|---|---|---|---|---|
|
|
||||
| Concentrates (9.2%) | Roughage (3.9%) | TMR (86.9%) | Phytoncide additive (Vehicle) | |
| Ingredient (%) | ||||
| ŌĆāCanola seed meal | 9.00 | |||
| ŌĆāCoconut kernel meal | 6.00 | |||
| ŌĆāCorn | 20.00 | |||
| ŌĆāCorn gluten feed | 13.60 | 5.00 | ||
| ŌĆāDicalcium phospate | 0.20 | |||
| ŌĆāLimestone | 1.50 | |||
| ŌĆāMgO | 0.20 | |||
| ŌĆāMolasses | 6.00 | 1.50 | ||
| ŌĆāParm kernel meal | 14.20 | 1.00 | ||
| ŌĆāSalt | 0.50 | |||
| ŌĆāSesame seed meal | 2.00 | |||
| ŌĆāSodium bicarbonate | 0.40 | |||
| ŌĆāSoybean meal | 10.00 | 2.00 | ||
| ŌĆāTallow | 2.00 | |||
| ŌĆāTapioca | 3.00 | |||
| ŌĆāVitamin mineral premix1 | 0.40 | |||
| ŌĆāWheat middling | 11.00 | |||
| ŌĆāCanola seed meal | 9.00 | |||
| ŌĆāTall fescue hay | 100 | |||
| ŌĆāAlfalfa hay | 6.50 | |||
| ŌĆāBeet pulp | 2.50 | |||
| ŌĆāConcentrate | 18.00 | |||
| ŌĆāCorn flake | 9.00 | |||
| ŌĆāCotton seed | 5.00 | |||
| ŌĆāKlein grass hay | 5.00 | |||
| ŌĆāOaten hay | 13.00 | |||
| ŌĆāTimothy hay | 2.50 | |||
| ŌĆāRice bran | 60 | |||
| ŌĆāCorn grit | 40 | |||
| Chemical composition | ||||
| ŌĆāDM (%) | 88.72 | 92.36 | 62.46 | - |
| ŌĆāCrude protein (% of DM) | 19.6 | 5.62 | 9.61 | 11.26 |
| ŌĆāEther extract (% of DM) | 4.75 | 1.44 | 3.71 | 8.74 |
| ŌĆāCrude fiber (% of DM) | 8.54 | 39.34 | 15.68 | 4.78 |
| ŌĆāCrude ash (% of DM) | 8.46 | 5.11 | 4.86 | 4.50 |
| ŌĆāNDF (% of DM) | 25.66 | 72.96 | 29.52 | - |
| ŌĆāADF (% of DM) | 11.1 | 43.6 | 18.08 | - |
| ŌĆāCa (% of DM)( | 1.27 | 0.17 | 0.52 | 0.12 |
| ŌĆāP (% of DM) | 0.64 | 0.14 | 0.27 | 1.00 |
| ŌĆāNEL (Mcal/kg of DM)2 | 2.10 | 2.08 | 2.15 | 2.32 |
TMR, total mixed ration; DM, dry matter; NDF, neutral detergent fiber; ADF, acid detergent fiber; ME, metabolizable energy; NEL, net energy value of feeds for lactation.
1 Vitamin mineral premix: Vit. A 1,000,000 IU; Vit. D3 100,000 IU; Vit. E 25,000 mg; I 150 mg; Co 150 mg; Cu 2,500 mg; Fe 6,250 mg; Mn 16,000 mg; Zn 10,000 mg; Se 150 mg.
2 NEL, Mcal/kg of DM was estimated based on NRC (2001).
Table┬Ā2
Major composition of terpenes in phytoncide oil1
Table┬Ā3
Effect of dietary phytoncide on milk yield in dairy cows1
| Period (d) | Milk yield (kg) | |
|---|---|---|
|
|
||
| C2 | T | |
| 0 | 34.6┬▒0.75 | 35.1┬▒1.87 |
| 10 | 34.0┬▒1.00 | 35.9┬▒1.60 |
| 20 | 34.4┬▒0.85 | 35.3┬▒1.37 |
| 30 | 34.0┬▒1.11 | 33.3┬▒1.52 |
| 0ŌĆō10 | 34.5┬▒0.88 | 35.4┬▒1.57 |
| 11ŌĆō20 | 34.1┬▒0.86 | 34.4┬▒1.29 |
| 21ŌĆō30 | 33.3┬▒1.11 | 34.3┬▒1.49 |
| 1ŌĆō30 | 33.9┬▒0.90 | 34.7┬▒1.42 |
Table┬Ā4
Effect of dietary phytoncide on milk composition in dairy cows1
| Item | Day | C2 | T |
|---|---|---|---|
| Milk fat (%, kg/d) | 30 | 4.1┬▒0.17 | 4.0┬▒0.12 |
| (1.4┬▒0.06) | (1.3┬▒0.04) | ||
| 1 to 30 | 4.1┬▒0.09 | 4.2┬▒0.10 | |
| (1.4┬▒0.04) | (1.5┬▒0.06) | ||
| Milk protein (%, kg/d) | 30 | 3.1┬▒0.09 | 3.1┬▒0.07 |
| (1.1┬▒0.03) | (1.0┬▒0.03) | ||
| 1 to 30 | 3.1┬▒0.04 | 3.0┬▒0.03 | |
| (1.1┬▒0.03) | (1.1┬▒0.04) | ||
| Lactose (%, kg/d) | 30 | 4.8┬▒0.05 | 4.8┬▒0.04 |
| (1.6┬▒0.05) | (1.6┬▒0.05) | ||
| 1 to 30 | 4.7┬▒0.02 | 4.8┬▒0.03* | |
| (1.6┬▒0.04) | (1.7┬▒0.07) | ||
| SnF (%, kg/d) | 30 | 8.5┬▒0.09 | 8.6┬▒0.08 |
| (2.9┬▒0.09) | (2.8┬▒0.09) | ||
| 1 to 30 | 8.5┬▒0.05 | 8.5┬▒0.04 | |
| (2.9┬▒0.08) | (3.0┬▒0.12) | ||
| Somatic cells (1,000/mL) | 30 | 59.0┬▒8.25 | 83.7┬▒28.77 |
| 1 to 30 | 134.7┬▒46.33 | 136.0┬▒33.84 | |
| MUN (mg/dL) | 30 | 18.7┬▒1.05 | 17.8┬▒0.69 |
| 1 to 30 | 18.2┬▒0.38 | 17.7┬▒0.35 | |
| Aceton (mM) | 30 | 0.1┬▒0.01 | 0.1┬▒0.01 |
| 1 to 30 | 0.1┬▒0.01 | 0.1┬▒0.01 | |
| BHB (mM) | 30 | 0 | 0 |
| 1 to 30 | 0 | 0 |
Table┬Ā5
Effect of dietary phytoncide on milk fatty acid contents in dairy cows1
| Fatty acids (% of total fatty acid) | C2 | T |
|---|---|---|
| C4:0 (Butryic) | 1.85┬▒0.038 | 1.92┬▒0.043 |
| C6:0 (Caproic) | 1.64┬▒0.037 | 1.65┬▒0.044 |
| C8:0 (Caprylic) | 1.07 ┬▒0.042 | 1.04 ┬▒0.052 |
| C10:0 (Capric) | 2.58 ┬▒0.140 | 2.42 ┬▒0.151 |
| C11:0 (Undecanoic) | 0.25 ┬▒0.018 | 0.23 ┬▒0.020 |
| C12:0 (Lauric) | 6.44 ┬▒0.239 | 5.78 ┬▒0.148* |
| C14:0 (Myristic) | 12.76 ┬▒0.281 | 12.19 ┬▒0.268 |
| C14:1 (Myristoleic) | 0.67 ┬▒0.052 | 0.65 ┬▒0.033 |
| C15:0 (Pentadecanoic) | 0.84 ┬▒0.032 | 0.81 ┬▒0.038 |
| C16:0 (Palmitic) | 29.90 ┬▒0.530 | 29.56 ┬▒0.310 |
| C16:1 (Palmitoleic) | 1.45 ┬▒0.091 | 1.36 ┬▒0.037 |
| C17:0 (Heptadecanoic) | 0.49 ┬▒0.007 | 0.50 ┬▒0.014 |
| C17:1 (cis-10-Heptadecenoic) | 0.09 ┬▒0.006 | 0.11 ┬▒0.008 |
| C18:0 (Stearic) | 14.25 ┬▒0.709 | 15.25 ┬▒0.497 |
| Trans vaccenic acid | 0.66 ┬▒0.035 | 0.73 ┬▒0.057 |
| C18:1n9c (Oleic) | 20.54 ┬▒0.503 | 21.28 ┬▒0.511 |
| C18:2n6t (Linolelaidic) | 0.23 ┬▒0.023 | 0.21 ┬▒0.016 |
| C18:2n6c (Linoleic) | 2.49 ┬▒0.110 | 2.42 ┬▒0.076 |
| C20:0 (Arachidic) | 0.25 ┬▒0.009 | 0.25 ┬▒0.007 |
| C18:3n6 (╬│-Linolenic) | 0.04 ┬▒0.001 | 0.04 ┬▒0.002 |
| C20:1n9 (cis-11-Eicosenoic) | 0.06 ┬▒0.008 | 0.06 ┬▒0.006 |
| C18:3n3 (╬▒-Linolenic) | 0.36 ┬▒0.014 | 0.36 ┬▒0.009 |
| cis-9, trans-11-CLA | 0.35 ┬▒0.016 | 0.45 ┬▒0.020* |
| C21:0(Henicosanoic) | 0.04 ┬▒0.001 | 0.04 ┬▒0.002 |
| trans-10, cis-12-CLA | 0.038 ┬▒0.0012 | 0.042 ┬▒0.0017* |
| C20:2 (cis-11,14-Eicosadienoic) | 0.05 ┬▒0.001 | 0.05 ┬▒0.002 |
| C22:0 (Behenic) | 0.05 ┬▒0.002 | 0.06 ┬▒0.004 |
| C20:3n6 (cis-8,11,14-Eicosatrienoic) | 0.15 ┬▒0.005 | 0.16 ┬▒0.003 |
| C22:1n9 (Erucic) | 0.09 ┬▒0.002 | 0.08 ┬▒0.006 |
| C20:4n6 (Arachidonic) | 0.16 ┬▒0.008 | 0.14 ┬▒0.016 |
| C23:0 (Tricosanoic) | 0.04 ┬▒0.001 | 0.04 ┬▒0.002 |
| C22:2 (cis-13,16-Docosadienoic) | 0.038 ┬▒0.0012 | 0.042 ┬▒0.0016* |
| C20:5n3 (cis-5,8,11,14,17-Eicosatrienoic) | 0.040 ┬▒0.0013 | 0.045 ┬▒0.0021* |
| C24:1n9 (Nervonic) | 0.04 ┬▒0.001 | 0.04 ┬▒0.002 |
| Total % | 100 | 100 |
| CLA | 0.39 ┬▒0.017 | 0.50 ┬▒0.020* |
| Žē3 | 0.40 ┬▒0.014 | 0.41 ┬▒0.009 |
| Žē6 | 3.07 ┬▒0.102 | 2.97 ┬▒0.081 |
| Žē6/Žē3 | 7.72 ┬▒0.190 | 7.25 ┬▒0.115* |
Table┬Ā6
Effect of dietary phytoncide on complete blood count analysis in dairy cows1
| Item | C2 | T |
|---|---|---|
| WBC, 4 to 12 K/╬╝L3 | 8.67┬▒0.57 | 10.15┬▒0.68 |
| Lymphocyte, 2.5 to 7.5 K/╬╝L | 5.62┬▒0.45 | 6.67┬▒0.39 |
| Monocyte, 0 to 0.84 K/╬╝L | 0.78┬▒0.09 | 0.97┬▒0.07 |
| Granulocyte, 0.6 to 6.7 K/╬╝L | 2.33┬▒0.19 | 2.41┬▒0.42 |
| RBC, 5 to 10 M/╬╝L | 6.41┬▒0.23 | 6.44┬▒0.24 |
| Hemoglobin, 8 to 15 g/dL | 11.81┬▒0.37 | 11.86┬▒0.35 |
| Hematocrit, 24% to 46% | 30.20┬▒0.68 | 30.47┬▒0.84 |
| MCV, 40 to 60 fL | 47.40┬▒1.28 | 47.70┬▒1.45 |
| MCH, 11 to 17 pg | 18.63┬▒0.85 | 18.37┬▒0.52 |
| MCHC, 30 to 36 g/dL | 39.19┬▒1.30 | 38.60┬▒0.82 |
| Platelet, 100 to 800 K/╬╝L | 203.40┬▒26.52 | 177.40┬▒13.92 |
REFERENCES
Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. 2006. Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem 17:789ŌĆō810.


Burnett TA, Madureira AML, Silper BF, Nadalin A, Tahmasbi A, Veira DM, Cerri RLA. 2014. Short communication: Factors affecting hair cortisol concentrations in lactating dairy cows. J Dairy Sci 97:7685ŌĆō7690.


Carrillo-Dominguez S, Carranco-Jauregui ME, Castillo-Dominguez RM, Castro-Gonzalez MI, Avila-Gonzalez E, Perez-Gil F. 2005. Cholesterol and n-3 and n-6 fatty acid content in eggs from laying hens fed with red crab meal (pleuroncodes planipes). Poult Sci 84:167ŌĆō172.


Concha C, Hu S, Holmberg O. 1996. The proliferative responses of cow stripping milk and blood lymphocytes to pokeweed mitogen and ginseng in vitro. Vet Res 27:107ŌĆō115.

Connor WE. 2000. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr 71:171SŌĆō175S.


Corl BA, Baumgard LH, Dwyer DA, Griinari JM, Phillips BS, Bauman DE. 2001. The role of delta (9)-desaturase in the production of cis-9, trans-11 cla. J Nutr Biochem 12:622ŌĆō630.


Dhiman TR, Satter LD, Pariza MW, Galli MP, Albright K, Tolosa MX. 2000. Conjugated linoleic acid (CLA) content of milk from cows offered diets rich in linoleic and linolenic acid. J Dairy Sci 83:1016ŌĆō1027.


Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497ŌĆō509.


Griinari JM, Corl BA, Lacy SH, Chouinard PY, Nurmela KVV, Bauman DE. 2000. Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by delta (9)-desaturase. J Nutr 130:2285ŌĆō2291.


Harfoot CG, Hazlewood GP. 1997. Lipid metabolism in the rumen. The Rumen Microbial Ecosystem. Hobson PN, Stewart CS, editorsSpringer; Netherlands: p. 382ŌĆō426.

Helander IM, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LGM, von Wright A. 1998. Characterization of the action of selected essential oil components on gram-negative bacteria. J Agric Food Chem 46:3590ŌĆō3595.

Hosoda K, Nishida T, Park WY, Eruden B. 2005. Influence of mentha├Śpiperita L. (peppermint) supplementation on nutrient digestibility and energy metabolism in lactating dairy cows. Asian Australas J Anim Sci 18:1721ŌĆō1726.

Igal RA. 2011. Roles of stearoylCoA desaturase-1 in the regulation of cancer cell growth, survival and tumorigenesis. Cancers 3:2462ŌĆō2477.



Kay JK, Mackle TR, Auldist MJ, Thomson NA, Bauman DE. 2004. Endogenous synthesis of cis-9, trans-11 conjugated linoleic acid in dairy cows fed fresh pasture. J Dairy Sci 87:369ŌĆō378.


Khiaosa-ard R, Zebeli Q. 2013. Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants. J Anim Sci 91:1819ŌĆō1830.


Kim YJ, Liu RH, Bond DR, Russell JB. 2000. Effect of linoleic acid concentration on conjugated linoleic acid production by butyrivibrio fibrisolvens A38. Appl Environ Microbiol 66:5226ŌĆō5230.



Lee Y, Vanden Heuvel JP. 2010. Inhibition of macrophage adhesion activity by 9trans,11trans-conjugated linoleic acid. J Nutr Biochem 21:490ŌĆō497.


Lefcourt AM, Bitman J, Kahl S, Wood DL. 1993. Circadian and ultradian rhythms of peripheral cortisol concentrations in lactating dairy cows. J Dairy Sci 76:2607ŌĆō2612.


Li Q. 2010. Effect of forest bathing trips on human immune function. Environ Health Prev Med 15:9ŌĆō17.



Moloney F, Toomey S, Noone E, Nugent A, Allan B, Loscher CE, Roche HM. 2007. Antidiabetic effects of cis-9, trans-11-conjugated linoleic acid may be mediated via anti-inflammatory effects in white adipose tissue. Diabetes 56:574ŌĆō582.


NRC (National Research Council). 2001. Nutrient Requirements of Dairy Cattle. 7th rev ednNational Academy Press; Washington, DC, USA:
Simopoulos AP. 2010. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med 235:785ŌĆō795.

Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. 2010. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr 91:502ŌĆō509.



- TOOLS





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





