 |
 |
| Anim Biosci > Volume 28(12); 2015 > Article |
|
Abstract
The in vitro maturation (IVM) efficiency of porcine embryos is still low because of poor oocyte quality. Although brilliant cresyl blue positive (BCB+) oocytes with low glucose-6-phosphate dehydrogenase (G6PDH) activity have shown superior quality than BCB negative (−) oocytes with high G6PDH activity, the use of a BCB staining test before IVM is still controversial. This study aimed to shed more light on the subcellular characteristics of porcine oocytes after selection using BCB staining. We assessed germinal vesicle chromatin configuration, cortical granule (CG) migration, mitochondrial distribution, the levels of acetylated lysine 9 of histone H3 (AcH3K9) and nuclear apoptosis features to investigate the correlation between G6PDH activity and these developmentally related features. A pattern of chromatin surrounding the nucleoli was seen in 53.0% of BCB+ oocytes and 77.6% of BCB+ oocytes showed peripherally distributed CGs. After IVM, 48.7% of BCB+ oocytes had a diffused mitochondrial distribution pattern. However, there were no significant differences in the levels of AcH3K9 in the nuclei of blastocysts derived from BCB+ and BCB− oocytes; at the same time, we observed a similar incidence of apoptosis in the BCB+ and control groups. Although this study indicated that G6PDH activity in porcine oocytes was correlated with several subcellular characteristics such as germinal vesicle chromatin configuration, CG migration and mitochondrial distribution, other features such as AcH3K9 level and nuclear apoptotic features were not associated with G6PDH activity and did not validate the BCB staining test. In using this test for selecting porcine oocytes, subcellular characteristics such as the AcH3K9 level and apoptotic nuclear features should also be considered. Adding histone deacetylase inhibitors or apoptosis inhibitors into the culture medium used might improve the efficiency of IVM of BCB+ oocytes.
Glucose-6-phosphate dehydrogenase (G6PDH) activity decreases with oocyte maturation (Alm et al., 2005; Bhojwani et al., 2007). A brilliant cresyl blue (BCB) staining test can show different intracellular activity of G6PDH in oocytes because the G6PDH enzyme converts BCB from blue to a colorless state. Those oocytes still undergoing growth have high levels of G6PDH, so BCB is made colorless while fully-grown oocytes with superior developmental competence have a low amount of G6PDH, and BCB remains blue (Tiffin et al., 1991). Thus, the G6PDH level is an indirect marker of oocyte growth. Therefore, compared with conventional morphological selection methods, BCB staining seems to be a reliable test for selecting mature porcine oocytes. Earlier studies have shown that this test can select oocytes with superior quality in some species such as the pig, goat, bovine, mouse, and dog in terms of nuclear maturation, cleavage rate and blastocyst yield (Ericsson et al., 1993; Rodríguez-González et al., 2003; Wu et al., 2007; Rodrigues et al., 2009; Mirshamsi et al., 2013). However, the predictive value of the BCB staining test is still controversial (Kempisty et al., 2011; Pawlak et al., 2011a; Opiela and Katska-Ksiazkiewicz, 2013; Pereira et al., 2014; Pawlak et al., 2014). For example, oocytes derived from prepubertal animals performed poorly even though these oocytes were BCB+; only 12% and 4% of BCB+ oocytes derived from prepubertal heifers and prepubertal goats formed blastocysts, respectively (Rodríguez-González et al., 2002; Pujol et al., 2004). This suggests that not all BCB+ oocytes constitute a homogeneous population in terms of developmental competence.
Chromatin configuration is correlated with oocyte growth and the distributions of organelles such as cortical granules (CGs) and mitochondria are also objective indicators of cytoplasmic maturation. The levels of acetylated lysine 9 of histone H3 (AcH3K9) and apoptosis reflect the developmental potential of embryos. Date are scarce concerning these subcellular characteristics in porcine oocytes after selecting with BCB staining, so the significance of these features among BCB+, BCB−, and control oocytes should be clarified. We investigated several such factors including germinal vesicle (GV) chromatin configuration, CG migration, mitochondrial distribution, AcH3K9 levels, and apoptotic nuclear features of BCB+, BCB−, and control oocytes. These studies should clearly characterize BCB+, BCB−, and control porcine oocytes in terms of organelle features and contribute to the establishment of better in vitro maturation (IVM) systems for porcine BCB+ oocytes.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated.
Porcine ovaries were collected at a local abattoir and transported to the laboratory in 0.9% NaCl solution at 35ºC. Cumulus-oocyte complexes (COCs) were aspirated from 3 to 6 mm diameter antral follicles by using a 20 mL disposable syringe with an 18-gauge needle. After being collected, COCs were washed three times in Dulbecco’s phosphate buffered saline (DPBS) supplemented with 13 μmol/L of BCB and exposed to 13 μmol/L of BCB diluted in DPBS for 90 min at 38.5ºC in a humidified air atmosphere. Following BCB exposure, the COCs were then transferred to DPBS and washed twice. COCs were then examined under a stereomicroscope and divided into two groups: BCB+ (coloured cytoplasm, low G6PDH) and BCB− (colourless cytoplasm, increased G6PDH), respectively. COCs untreated with BCB were used as control.
COCs were denuded of cumulus cells by vortexing in the presence of 0.1% hyaluronidase in DPBS. The denuded oocytes were cultured in tissue culture medium (TCM) 199 containing 10 μg/mL Hoechst 33,342 for 30 min in humidified air. Oocytes of the same group were then placed on a glass slide and squashed with coverslips to visualize the GV. The GVs were examined by fluorescence optics. The GV chromatin configuration of oocytes was classified into the non-surrounded nucleoli (NSN) pattern with diffuse chromatin throughout the nucleus and the surrounded nucleoli (SN) pattern with chromatin condensed into a perinucleolar rim.
After being selected with BCB staining, COCs with an evenly distributed cytoplasm and at least three compact layers of cumulus cells were selected and washed three times in TCM 199 enriched with 10% (v/v) fetal bovine serum, and then were washed twice with the maturation medium (TCM 199 supplemented with 0.1% poly vinyl alcohol (PVA), 3.05 mM D-glucose, 0.91 mM sodium pyruvate, 0.57 mM cysteine, 10 IU/mL human chorionic gonadotrop, 10 IU/mL pregnant mare serum gonadotropin, 10 ng/mL epidermal growth factor, 10% porcine follicular fluid, 75 μg/mL penicillin G, and 50 μg/mL streptomycin). After about 21 h of culture with maturation medium, oocytes were cultured without hormones for another 21 h at 38.5ºC, 5% CO2. After IVM, COCs from each BCB+, BCB−, and control group were freed from surrounding cumulus cells and oocytes with the first polar body (finished nuclear maturation) were evaluated.
After denuded of cumulus cells, oocytes were fixed with 4% (w/v) paraformaldehyde in PBS for 30 min at 20ºC. After fixation, oocytes were rinsed with blocking solution (PBS containing 0.3% BSA and 100 mM glycine and 100 mM glycine) for 5 min, then were permeabilized with 0.1% Triton X-100 in PBS for 5 min. After being washed twice in blocking solution, the oocytes were then labeled with 100 mg/mL fluorescein isothiocyanate-labeled peanut agglutinin in PBS for 30 min in a dark box. Finally, the oocytes were rinsed thoroughly with PBS-PVA. The oocytes from each sample were mounted between a slide and coverslip, then scanned and recorded with confocal fluorescent microscopy.
After maturation, oocytes were transferred into denuding medium with hyaluronidase in a 1.5-mL centrifuge tube. The oocytes were vigorously vortexed for 4 to 5 min, then transferred into manipulation medium. Oocytes with an intact plasma membrane and the first polar body were stained with Mito-Tracker Red CMXRos (Invitrogen, Eugene, Oregon, USA). The dye was used at a concentration of 250 nM in TCM 199 for 30 min at 38.5ºC. After staining, oocytes were briefly rinsed in DPBS and fixed in 4% paraformaldehyde in DPBS for 30 min at 38.5ºC. The oocytes from each sample were mounted between a slide and coverslip, then scanned and recorded with confocal fluorescent microscopy. With regard to mitochondrion distribution, oocytes with diffuse mitochondrion throughout the entire cytoplasm were classified as mature and those with peripheral and semiperipheral mitochondrion as immature.
Only denuded oocytes with the first polar body were selected. For activation, 1 DC pulse of 2.0 kV/cm for 30 μs was applied, by using a BTX-Cell Manipulator 2001 (Genetronics, San Diego, CA, USA). The medium used for activation was 0.3 M mannitol supplemented with 0.1% PVA, 0.1 mM CaCl2, 0.1 mM MgCl2 and 0.5 mM Hepes. Then embryos were treated with 2 mM 6-DMAP (4-dimethylaminopyridine) for 3 h. Activated embryos were cultured in PZM-3 medium then examined for cleavage at 44 h after activation. The percentage of blastocyst derived from BCB+, BCB−, and control oocytes were determined on day 7.
For day 7 embryos derived from parthenogenetically activated (PA), zonae pellucidae were removed by pronase treatment. Blastocysts were washed in PBS, then fixed for 15 min in 4% paraformaldehyde in PBS, and embryos were permeabilized with 0.2% Triton X-100 in PBS-PVA for 30 min. Samples were blocked overnight at 4ºC in blocking solution. The samples were incubated with a rabbit polyclonal antibody to AcH3K9 (Millipore, Darmstadt, Germany ) diluted 1:200 for 12 h at 4ºC. After extensive washing, samples were incubated with a secondary antibody of Alexa Fluor 594-labeled goat anti-rabbit IgG (Invitrogen, Eugene, Oregon, USA) for AcH3K9. After washing three times, blastocysts were stained with Hoechst 33342 and mounted on slides. Fluorescence was observed under a fluorescence digital microscope (Axiovert 200M, ZEISS, Shanghai, China), and the fluorescence intensity of each pixel was transformed to obtain a quantitative measure using Image-J software. Quantification of AcH3K9 signal intensities in BCB+ and BCB− groups was expressed relative to that of the control embryos (set as 100%). Quantification of the AcH3K9 is represented as the mean±standard error (SE). Values with different superscripts means significant difference (p<0.05).
Apoptosis assays were carried out using a DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, USA). After being washed and fixed, the blastocyst was permeabilized for staining. After equilibration, embryos were incubated with terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) reaction mixture in the dark box for 1 h at 37ºC. The reaction was terminated in 2× SSC for 15 min. Finally, the DNA was stained with Hoechst 33342. Samples were mounted on glass slides for apoptosis assays. Blastocysts that served as a positive control were incubated in DNase I solutions (5 U/50 μL).
Following BCB exposure, the oocytes were examined under a stereomicroscope and classed as BCB+ (blue cytoplasm) and BCB− (colorless cytoplasm) (Figure 1). Figure 1 also shows two kinds of chromatin configuration we defined as SN and NSN. While most of the BCB− oocytes had an NSN configuration, more BCB+ oocytes were of the SN pattern (Figure 2). This result suggests that chromatin configuration patterns were associated with G6PDH activity in oocytes.
After 42 h in IVM medium, oocytes were denuded of cumulus cells. Only those oocytes with the first polar body extruded were regarded as having finished nuclear maturation (Figure 3). In total, 236 BCB+, 331 BCB−, and 303 control oocytes were used to determine nuclear status. Significantly more BCB+ oocytes had finished nuclear maturation than in the BCB− and control groups (p<0.05), with BCB− oocytes having the lowest percentage of nuclear maturation (Figure 4). This suggests that G6PDH activity was inversely associated with maturation of porcine oocytes.
CGs migration patterns in BCB+, BCB−, and control oocytes were evaluated under a confocal microscope. Figure 5 shows the two categories of CG migration patterns. Oocytes with CGs arranged throughout the entire cytoplasm were classified as immature and those with peripheral CGs as mature. A peripheral distribution was significantly more prevalent in BCB+ oocytes than in BCB− and control oocytes (p<0.05) (Figure 6). Thus, there was a negative association between G6PDH activity and the incidence of mature oocytes with peripheral CGs.
The mitochondrial distributions in BCB+, BCB−, and control oocytes were evaluated under a confocal microscope. Figure 7 shows the three categories of distribution. The peripheral pattern showed fluorescent signals distributed in the periphery of the oocyte; the semiperipheral pattern showed fluorescent signals covering most of the cytoplasmic volume without its central part; the diffuse pattern showed mitochondria distributed homogeneously throughout the ooplasm. Oocytes with the diffuse pattern were classified as mature and those with peripheral and semiperipheral mitochondrial distributions as immature. As expected, a higher proportion of oocytes with the diffuse pattern were found in BCB+ oocytes after IVM (p<0.05) (Figure 8).
PA embryos derived from BCB+, BCB−, and control oocytes were examined for cleavage at 48 h after activation. The percentages of blastocysts derived from BCB+, BCB−, and control oocytes were determined on day 7. Regarding the rate of cleavage, there were no significant differences among the BCB+, BCB−, and control groups. A significantly higher fraction of BCB+ oocytes developed into blastocysts compared with BCB− oocytes (p<0.05) whereas no significant difference was observed between the BCB+ and control groups (Table 1).
No signals could be detected in blastocysts without using primary or secondary antibodies, indicating specificity of staining by the primary antibody (data not shown). The global AcH3K9 in the nuclei of blastomeres derived from BCB+ and BCB− oocytes were compared, but no significant difference was observed (Figure 9).
BCB+, BCB−, and control groups were subjected to TUNEL analysis because the apoptotic index is another criterion for blastocyst quality. A blastocyst was scored as TUNEL positive when distinct, green fluorescence signals were observed. Although the BCB− group showed a higher apoptotic index, we observed a similar low incidence of apoptosis in BCB+ and control groups (Figure 10).
The BCB staining test is claimed to be an efficient method to select porcine oocytes of high quality. However, several studies have questioned the reliability of BCB staining test for routine IVM. The lack of significant differences in blastocyst formation rates between BCB+ and control groups led Opiela et al. (2008) to consider the BCB staining test questionable. Pawlak et al. (2014) found a high incidence of apoptosis and major variations in the diameter of BCB+ oocytes, making the BCB staining test a less effective selection tool than reported previously. Moreover, the BCB+ oocytes derived from pre-pubertal females and cycling gilts also differed in many aspects (Pawlak et al., 2011a,b). Overall, the quality of oocytes is a complex trait, and these ambivalent phenomena might arise from heterogeneity in BCB+ porcine oocytes. We need to evaluate the relationship between G6PDH activity and several subcellular characteristics in porcine oocytes after BCB staining selection to identify such heterogeneity. Here we investigated several subcellular characteristics such as GV chromatin configuration, CG migration, mitochondrial distribution, AcH3K9 level, and apoptotic nuclear features after BCB staining.
Chromatin condensation is associated with oocyte maturation and highly competent porcine oocytes have a more condensed chromatin configuration (Sun et al., 2004). Moreover, mouse oocytes with an SN configuration were more competent for development than oocytes with an NSN pattern (Wu et al., 2007). This suggests that the SN configuration in oocytes represents a stage toward ovulation and completion of oocyte growth (Guthrie and Garrett, 2000). In the present study, the percentage of the SN pattern in BCB+ oocytes was significantly higher than in control and BCB− oocytes. This indicated that BCB+ oocytes might have higher developmental potential in terms of chromatin configuration.
Organelle distributions are also objective indicators of cytoplasmic maturation in oocytes. During maturation, the migration of CGs to the cortex relies on microfilament assembly, and a peripheral CG distribution is one indication of oocyte maturity. We found an increased rate of cytoplasm maturation for the BCB+ group (77.6%) characterized by peripheral CG migration compared with the control groups (61.7%) and the BCB−groups (39.6%). Delays in CG migration during maturation could be caused by an intrinsic deficiency in BCB− oocytes (Silva et al., 2013). Unlike CGs, mitochondria are mostly concentrated in the periphery of the oocyte at the GV stage, indicating contact with cumulus cell cytoplasmic projections. They then migrate toward the center during maturation (Sun et al., 2001). A homogeneous distribution of mitochondria in the cytoplasm is positively associated with adenosine triphosphate content and reflects changes in energy requirement accompanying cytoplasmic maturation, which directly influences fertilization outcomes in the pig (Yang et al., 2010). Our finding that the rate of BCB+ oocytes with a diffuse mitochondrial distribution was significantly higher than in the other groups also indicated a higher level of cytoplasmic maturation and stronger developmental potential.
We did not find a significant difference between the blastocyst formation rates of BCB+ and the control group in this study. This result is consistent with other reports (Opiela et al., 2008; Ishizaki et al., 2009) and might have resulted from a prolonged incubation time during BCB staining. In addition, porcine oocytes are commonly recovered from the ovaries of slaughtered animals and oocytes may be at various stages of the estrous cycle. Thus, BCB+ oocytes derived from different stages of the estrous cycle (e.g., pre-pubertal females or cycling gilts) still differ in many aspects; oocytes from cycling gilts have higher developmental competence than those from pre-pubertal pigs, even though BCB+ oocytes predominated among the oocytes collected from both groups (Pawlak et al., 2011a). Thus, the heterogeneity of BCB+ porcine oocytes and a lack of significant difference between the blastocyst formation rates of BCB+ and control groups decreases the validity of the BCB staining test.
The AcH3K9 level and apoptotic nuclear features affect the developmental potential of embryos and the different G6PDH activities as measured by BCB staining could not be reflected by these two subcellular characteristics in this study. Acetylation levels of nuclear histones are major epigenetic modifications of the genome, which play important roles in the development of embryos (Dai et al., 2010; Das et al., 2010). To our knowledge, the effects of selecting porcine oocytes using the BCB staining test on histone modifications have not yet been investigated. The global acetylation levels of H3K9 were analyzed here. We found that the BCB+ and BCB− groups showed similar global levels of AcH3K9 at the blastocyst stage. This result is in agreement with an earlier report in cattle (Su et al., 2012). Given the similar acetylation levels of H3K9 between groups, the BCB staining test seems not to be valuable for evaluating oocyte quality for this factor. More studies measuring the acetylation level of other histone lysine residues would be helpful to verify BCB staining test further.
Although the level of apoptosis is another criterion of embryo quality, the value of measuring G6PDH activity as an indirect marker of apoptosis seems to be questionable. The lack of significant differences between the ratio of densitometric signals of Bax to Bcl-2 in BCB− and control immature oocytes or in BCB+, BCB−, and control mature oocytes suggested a lack of interaction between G6PDH activity and the expression of these apoptosis-related proteins (Opiela et al., 2008). Moreover, Pawlak found that BCB+ oocytes derived from ovaries of cycling gilts were more often apoptotic than BCB+ oocytes derived from ovaries of pre-pubertal pigs (Pawlak et al., 2011a). We found a similar incidence of apoptosis in BCB+ and control blastocyst groups in terms of DNA fragmentation measured by the TUNEL assay. These phenomena might arise from donor age, or the apoptotic changes might be a reflection of a better adaptability to potentially harmful conditions during prolonged BCB staining (Anguita et al., 2009; Pawlak et al., 2011a).
The value of the BCB staining test for assessing oocyte quality seems to be questionable according to AcH3K9 levels and apoptotic features. Although this study indicated that G6PDH activity in porcine oocytes was associated with several subcellular characteristics such as GV chromatin configuration, CG migration, and mitochondrial distribution, other factors including the AcH3K9 level and apoptosis rates were not. Our results show a high level of heterogeneity in BCB+ porcine oocytes in terms of the acetylation level of H3K9 and apoptotic traits. For future use, adding inhibitors of histone deacetylases or apoptosis to the culture medium used for IVM of BCB+ oocytes might improve the efficiency of the system.
ACKNOWLEDGMENTS
This work was partly supported by National Natural Science Foundation of China (No. 31201804, No. 31101700), Natural Science Foundation of HLJ province (No. QC2013C021), Postdoctoral Programme of HLJ province (No. LBr-211032) and Heilongjiang Academy of Agricultural Sciences Doctoral scientific research projects (201507-32).
Figure 1
Cumulus-oocyte complexes after brilliant cresyl blue (BCB) staining and germinal vesicle (GV) chromatin configurations of porcine oocytes. (A) The arrow indicates BCB+ oocyte with blue ooplasm, whereas colorless ooplasm indicates BCB− oocyte. Bar = 100 μm. The GV chromatin configuration of oocytes was stained by 10 μg/mL Hoechst 33342. (B) Non-surrounded nucleoli (NSN) pattern with diffuse chromatin throughout the nucleus. (C) Surrounded nucleoli (SN) pattern with chromatin condensed into a perinucleolar rim. Bar = 20 μm.
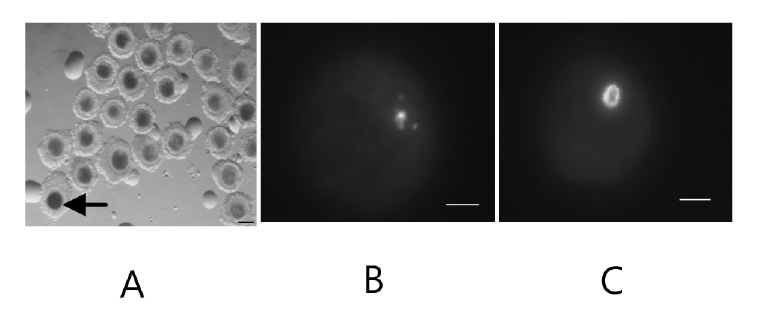
Figure 2
Germinal vesicle chromatin configurations of brilliant cresyl blue positive (BCB+), BCB−, and control oocytes. Different superscripts show statistically significant differences between oocytes from BCB+, BCB−, and control groups (p<0.05). The experiment was repeated for three times. SN, surrounded nucleoli.
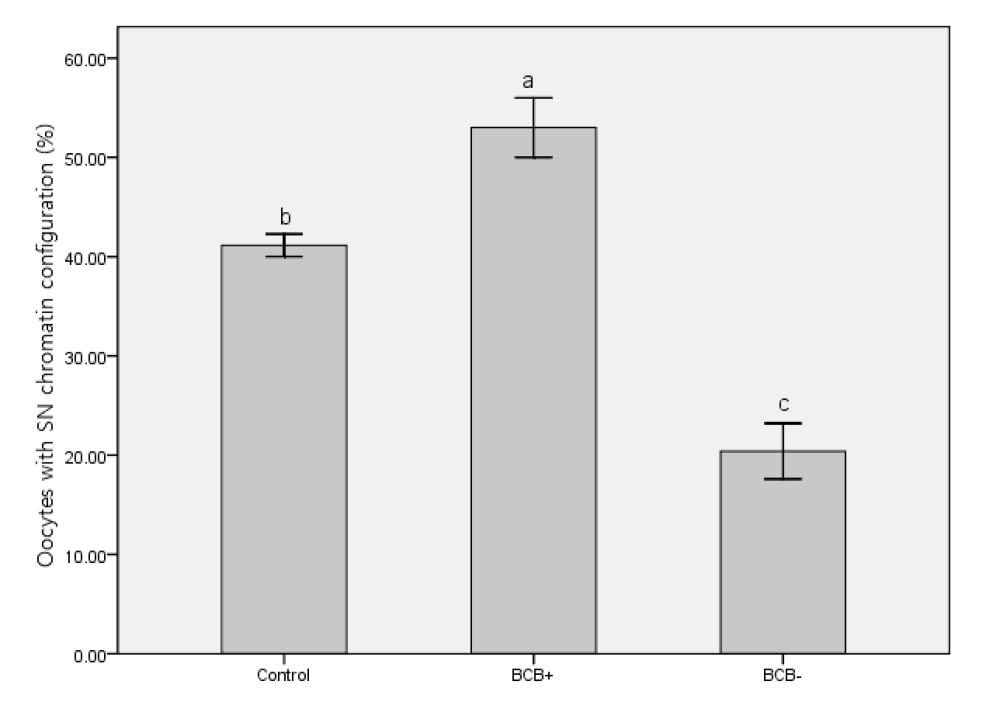
Figure 3
The mature oocyte with the first polar. Cumulus-oocyte complexes were freed from surrounding cumulus cells and oocytes with the first polar body (at MII) were evaluated. Bar = 100 μm.
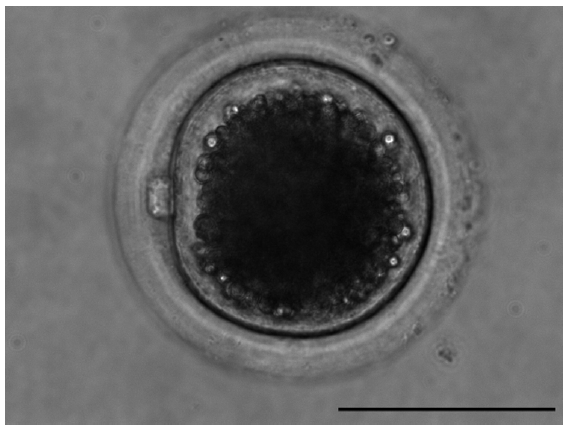
Figure 4
Nuclear maturation of brilliant cresyl blue positive (BCB+), BCB−, and control oocytes. Different superscripts show statistically significant differences between oocytes from BCB+, BCB−, and control groups (p<0.05). The experiment was repeated for three times.
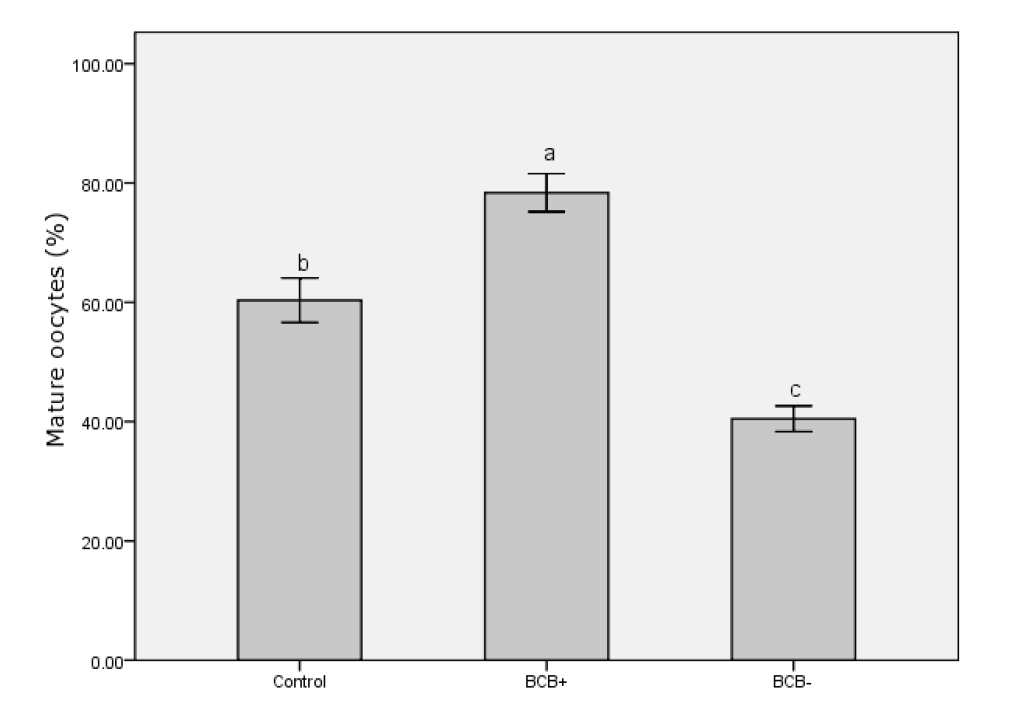
Figure 5
The confocal micrographs of cortical granules (CGs) migration in porcine oocytes. (A) CGs were distributed in the whole oocyte cytoplasma including the cortex. (B) CGs were distributed at the cortex and closely located beneath the membrane and formed a brilliant continuous ring. Bar = 50 μm.
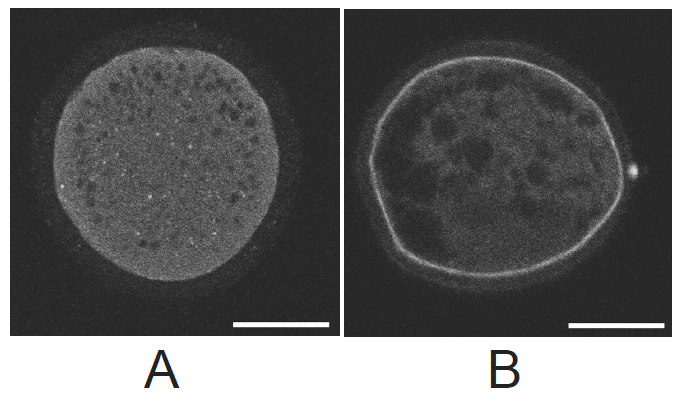
Figure 6
Cortical granules (CGs) migration of brilliant cresyl blue positive (BCB+), BCB−, control oocytes. Different superscripts show statistically significant differences between oocytes from BCB+, BCB−, and control groups (p<0.05). The experiment was repeated for three times.
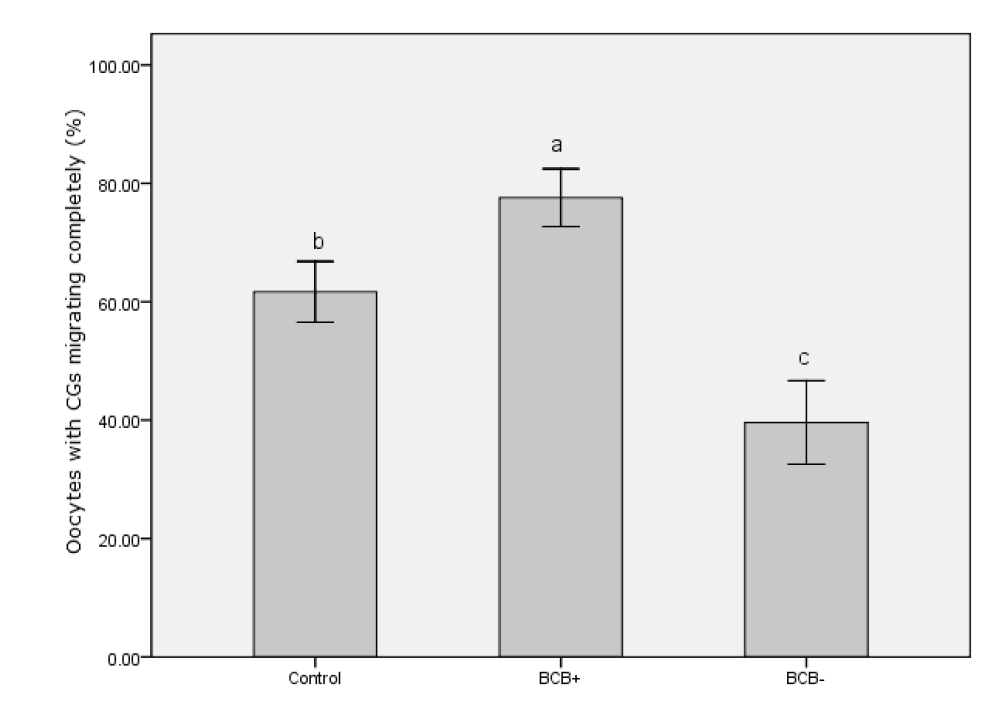
Figure 7
The confocal micrographs of mitochondrion distribution in porcine oocytes. (A) Fluorescent signals distributed in the peripheral (peripheral pattern). (B) Fluorescent signals covering most of the cytoplasmic volume without its central part (semiperipheral pattern). (C) Mitochondria distributed homogeneously throughout the ooplasm (diffuse pattern). Bar = 50 μm.
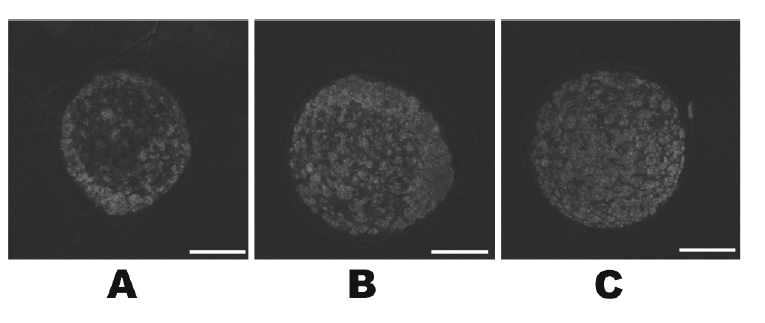
Figure 8
Mitochondrion distribution of brilliant cresyl blue positive (BCB+), BCB−, and control oocytes. Different superscripts show statistically significant differences between oocytes from BCB+, BCB−, and control groups (p<0.05). The experiment was repeated for three times.
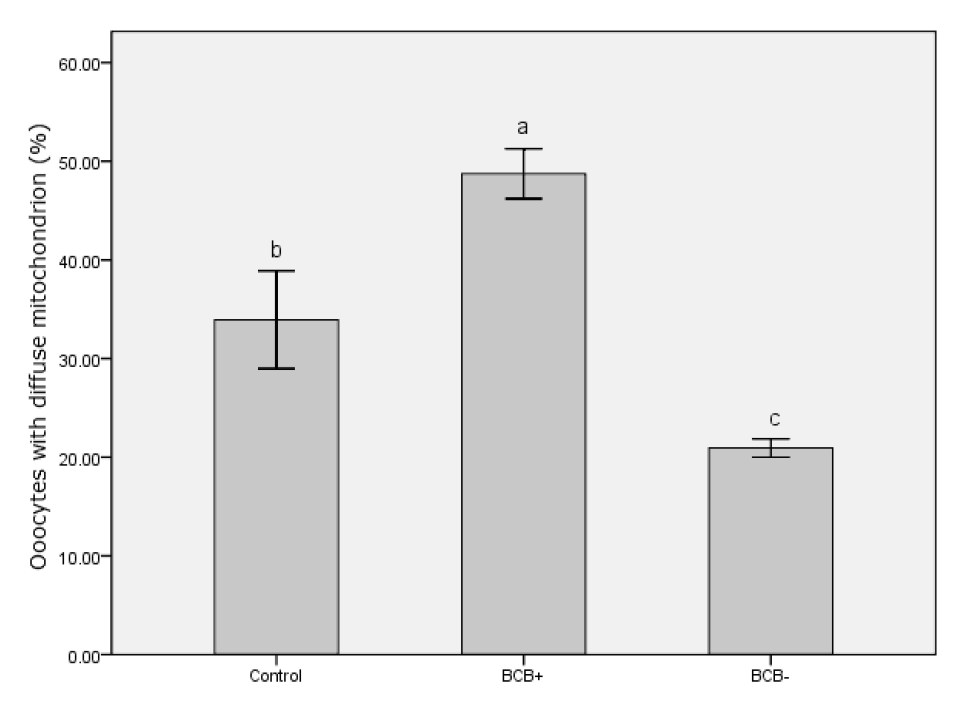
Figure 9
The acetylation levels of H3K9 (lysine 9 of histone H3) in blastocysts. (A) Staining of acetylated lysine 9 of histone H3 (AcH3K9) in control, brilliant cresyl blue positive (BCB+), BCB− groups at blastocyst stage was shown. Each sample was counterstained with Hoechst 33342 to visualize DNA (blue). Bar = 50 μm. (B) Quantification of AcH3K9 signal intensities in BCB+ and BCB− at blastocyst stage was expressed relative to that of the control blastocysts (set as 100%). The experiments were replicated three times. In each replication, n = 15 per group.
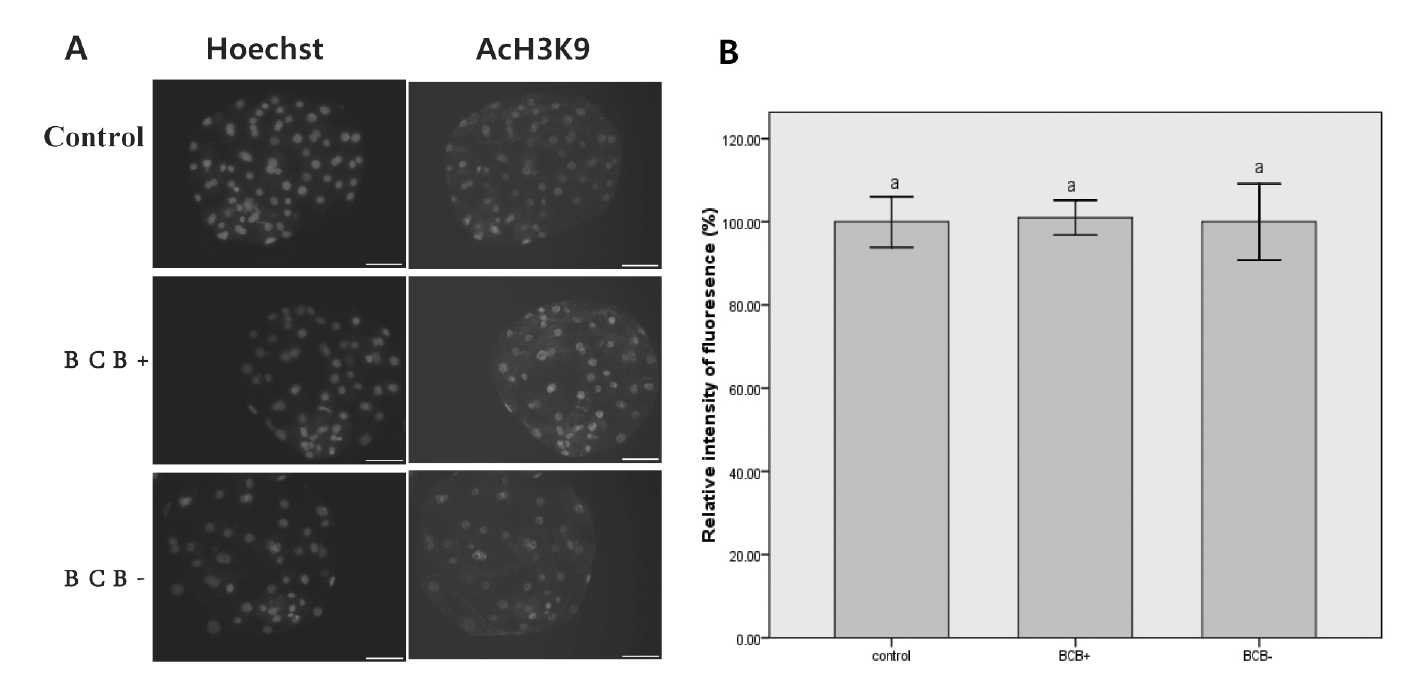
Figure 10
Incidence of apoptosis in blastocysts. (A) Representative photographs of terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) assay of blastocysts (green). Each sample was counterstained with Hoechst 33342 to visualize DNA (blue). Bar = 50 μm. (B) Number of apoptotic cells in each blastocyst. Values with different superscripts differ significantly (p<0.05). BCB, brilliant cresyl blue.
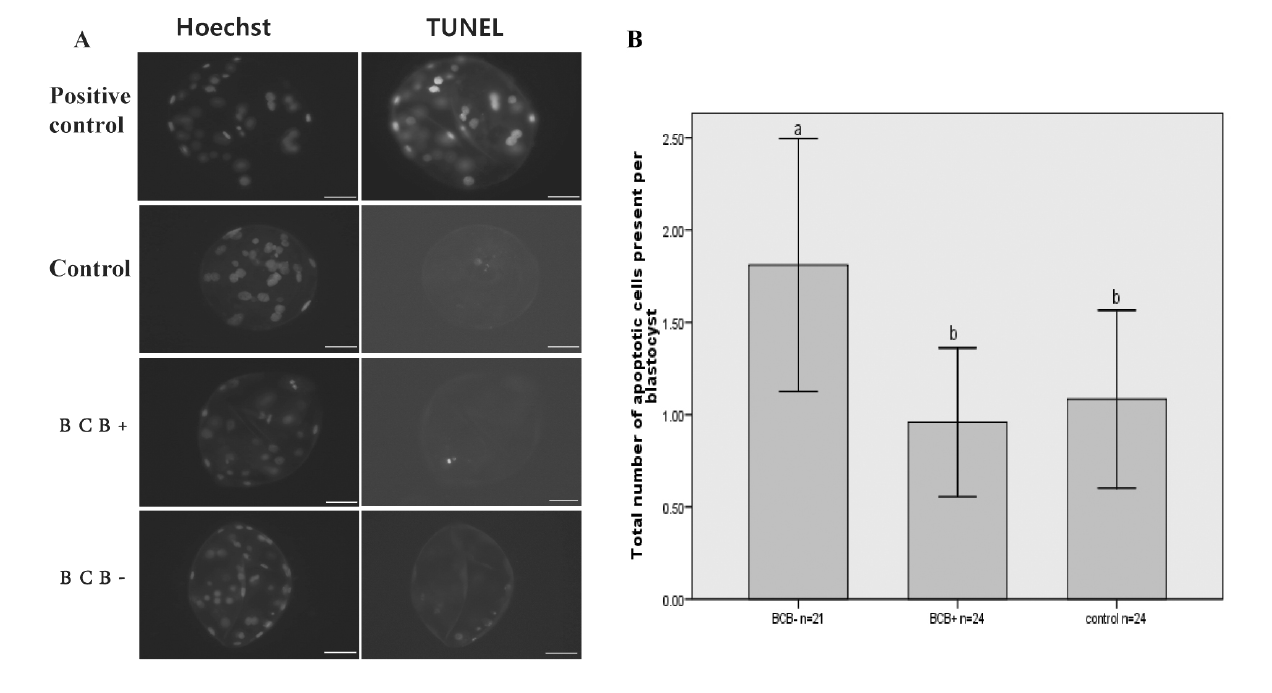
Table 1
Effect of G6PDH activity on the development of PA embryos
REFERENCES
Alm H, Torner H, Lohrke B, Viergutz T, Ghoneim IM, Kanitz W. 2005. Bovine blastocyst development rate in vitro is influenced by selection of oocytes by brillant cresyl blue staining before IVM as indicator for glucose-6-phosphate dehydrogenase activity. Theriogenology 63:2194–2205.


Anguita B, Paramio MT, Morato R, Romaguera R, Jimenez-Macedo AR, Mogas T, Izquierdo D. 2009. Effect of the apoptosis rate observed in oocytes and cumulus cells on embryo development in prepubertal goats. Anim Reprod Sci 116:95–106.


Bhojwani S, Alm H, Torner H, Kanitz W, Poehland R. 2007. Selection of developmentally competent oocytes through brilliant cresyl blue stain enhances blastocyst development rate after bovine nuclear transfer. Theriogenology 67:341–345.


Dai X, Hao J, Hou XJ, Hai T, Fan Y, Yu Y, Jouneau A, Wang L, Zhou Q. 2010. Somatic nucleus reprogramming is significantly improved by m-carboxycinnamic acid bishydroxamide, a histone deacetylase inhibitor. J Biol Chem 285:31002–31010.



Das ZC, Gupta MK, Uhm SJ, Lee HT. 2010. Increasing histone acetylation of cloned embryos, but not donor cells, by sodium butyrate improves their in vitro development in pigs. Cell Reprogram 12:95–104.


Ericsson SA, Boice ML, Funahashi H, Day BN. 1993. Assessment of porcine oocytes using brilliant cresyl blue. Theriogenology 39:214

Guthrie HD, Garrett WM. 2000. Changes in porcine oocyte germinal vesicle development as follicles approach preovulatory maturity. Theriogenology 54:389–399.


Ishizaki C, Watanabe H, Bhuiyan MM, Fukui Y. 2009. Developmental competence of porcine oocytes selected by brilliant cresyl blue and matured individually in a chemically defined culture medium. Theriogenology 72:72–80.


Kempisty B, Jackowska M, Piotrowska H, Antosik P, Wozna M, Bukowska D, Brussow KP, Jaskowski JM. 2011. Zona pellucida glycoprotein 3 (pZP3) and integrin beta2 (ITGB2) mRNA and protein expression in porcine oocytes after single and double exposure to brilliant cresyl blue test. Theriogenology 75:1525–1535.


Mirshamsi SM, Karamishabankareh H, Ahmadi-Hamedani M, Soltani L, Hajarian H, Abdolmohammadi AR. 2013. Combination of oocyte and zygote selection by brilliant cresyl blue (BCB) test enhanced prediction of developmental potential to the blastocyst in cattle. Anim Reprod Sci 136:245–251.


Opiela J, Kątska-Książkiewicz L. 2013. The utility of brilliant cresyl blue (BCB) staining of mammalian oocytes used for in vitro embryo production (IVP). Reprod Biol 13:177–183.


Opiela J, Kątska-Książkiewicz L, Lipinski D, Slomski R, Bzowska M, Rynska B. 2008. Interactions among activity of glucose-6-phosphate dehydrogenase in immature oocytes, expression of apoptosis-related genes Bcl-2 and Bax, and developmental competence following IVP in cattle. Theriogenology 69:546–555.


Pawlak P, Pers-Kamczyc E, Renska N, Kubickova S, Lechniak D. 2011a. Disturbances of nuclear maturation in BCB positive oocytes collected from peri-pubertal gilts. Theriogenology 75:832–840.


Pawlak P, Renska N, Pers-Kamczyc E, Warzych E, Lechniak D. 2011b. The quality of porcine oocytes is affected by sexual maturity of the donor gilt. Reprod Biol 11:1–18.


Pawlak P, Warzych E, Chabowska A, Lechniak D. 2014. Differences in cytoplasmic maturation between the BCB+ and control porcine oocytes do not justify application of the BCB test for a standard IVM protocol. J Reprod Dev 60:28–36.



Pereira GR, Lorenzo PL, Carneiro GF, Bilodeau-Goeseels S, Kastelic JP, Esteller-Vico A, Lopez-Bejar M, Liu IK. 2014. Selection of developmentally competent immature equine oocytes with brilliant cresyl blue stain prior to in vitro maturation with equine growth hormone. Zygote 22:500–504.


Pujol M, López-Béjar M, Paramio MT. 2004. Developmental competence of heifer oocytes selected using the brilliant cresyl blue (BCB) test. Theriogenology 61:735–744.


Rodrigues BA, Rodriguez P, Silva AE, Cavalcante LF, Feltrin C, Rodrigues JL. 2009. Preliminary study in immature canine oocytes stained with brilliant cresyl blue and obtained from bitches with low and high progesterone serum profiles. Reprod Domest Anim 44:Suppl 2255–258.

Rodríguez-González E, López-Bejar M, Izquierdo D, Paramio MT. 2003. Developmental competence of prepubertal goat oocytes selected with brilliant cresyl blue and matured with cysteamine supplementation. Reprod Nutr Dev 43:179–187.


Rodríguez-González E, López-Bejar M, Velilla E, Paramio MT. 2002. Selection of prepubertal goat oocytes using the brilliant cresyl blue test. Theriogenology 57:1397–1409.


Silva DS, Rodriguez P, Galuppo A, Arruda NS, Rodrigues JL. 2013. Selection of bovine oocytes by brilliant cresyl blue staining: effect on meiosis progression, organelle distribution and embryo development. Zygote 21:250–255.


Su J, Wang Y, Li R, Peng H, Hua S, Li Q, Quan F, Guo Z, Zhang Y. 2012. Oocytes selected using BCB staining enhance nuclear reprogramming and the in vivo development of SCNT embryos in cattle. PloS one 7:e36181



Sun QY, Wu GM, Lai L, Park KW, Cabot R, Cheong HT, Day BN, Prather RS, Schatten H. 2001. Translocation of active mitochondria during pig oocyte maturation, fertilization and early embryo development in vitro. Reproduction 122:155–163.


Sun XS, Liu Y, Yue KZ, Ma SF, Tan JH. 2004. Changes in germinal vesicle (GV) chromatin configurations during growth and maturation of porcine oocytes. Mol Reprod Dev 69:228–234.


Tiffin GJ, Rieger D, Betteridge KJ, Yadav BR, King WA. 1991. Glucose and glutamine metabolism in pre-attachment cattle embryos in relation to sex and stage of development. J Reprod Fertil 93:125–132.


- TOOLS
-
METRICS

- Related articles
-
Screening and Characterization of Lactate Dehydrogenase-producing Microorganism2004 January;17(10)





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




